New MDCG Guideline 2024-14 Rev.1

What Contact Lens Manufacturers Need to Know About the Master UDI-DI At the end of August 2025, the Medical Device Coordination Group (MDCG) published its revised guidance MDCG 2024-14 Rev.1.…

What Contact Lens Manufacturers Need to Know About the Master UDI-DI At the end of August 2025, the Medical Device Coordination Group (MDCG) published its revised guidance MDCG 2024-14 Rev.1.…
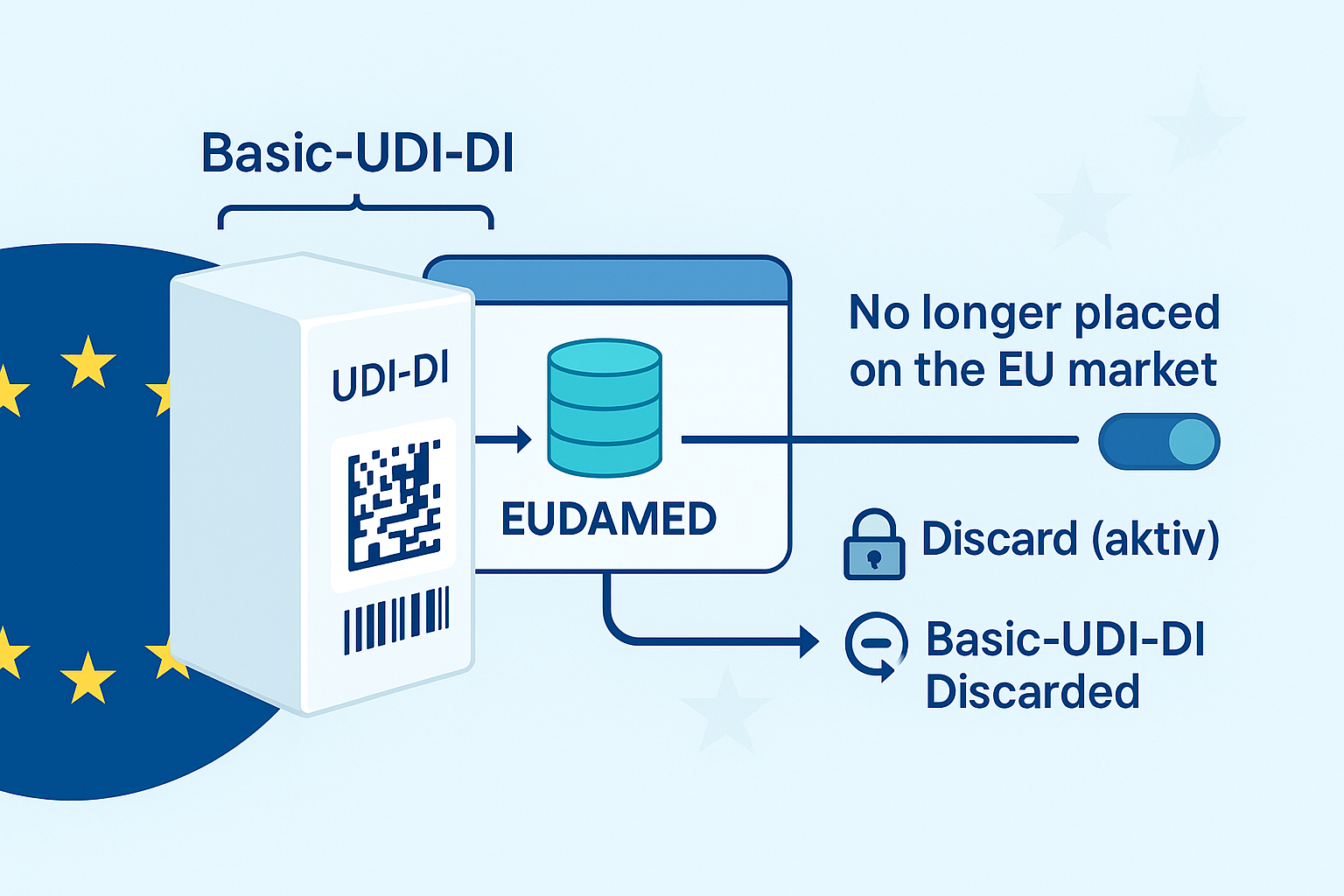
Basic UDI-DI in transition scenarios: Can a BUDI be "empty"? 1. Can a Basic UDI-DI (transitional) exist without associated UDI-DI(s)? No – in EUDAMED, a basic UDI-DI is not registered…

EU Launches "Apply AI": €1 Billion for AI in Key Industries – Focus on Healthcare The European Commission is launching "Apply AI", an investment package worth €1 billion, to accelerate…
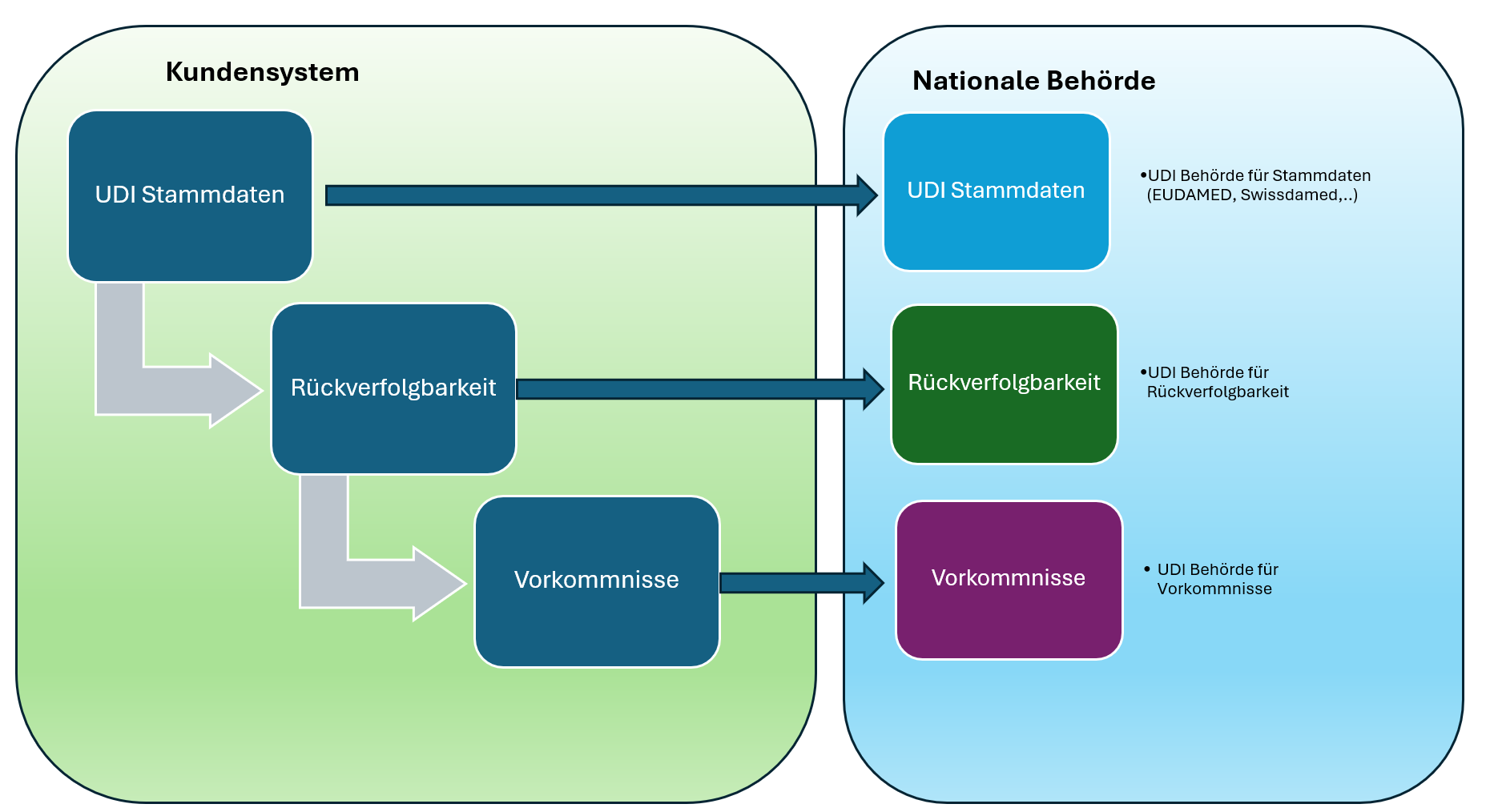
The full UDI lifecycle – everything from a single source The path to full UDI compliance can be understood as a data cycle: UDI master data → Traceability → Vigilance…

When does the UDI-DI status need to be changed?In EUDAMED, the status (not the country end data) decides whether a device is considered "On the EU market" or "No longer…
Do custom-made devices (CMD) have to be registered in EUDAMED – and what about cases of vigilance? What are custom-made products according to the MDR? Please also read our article…
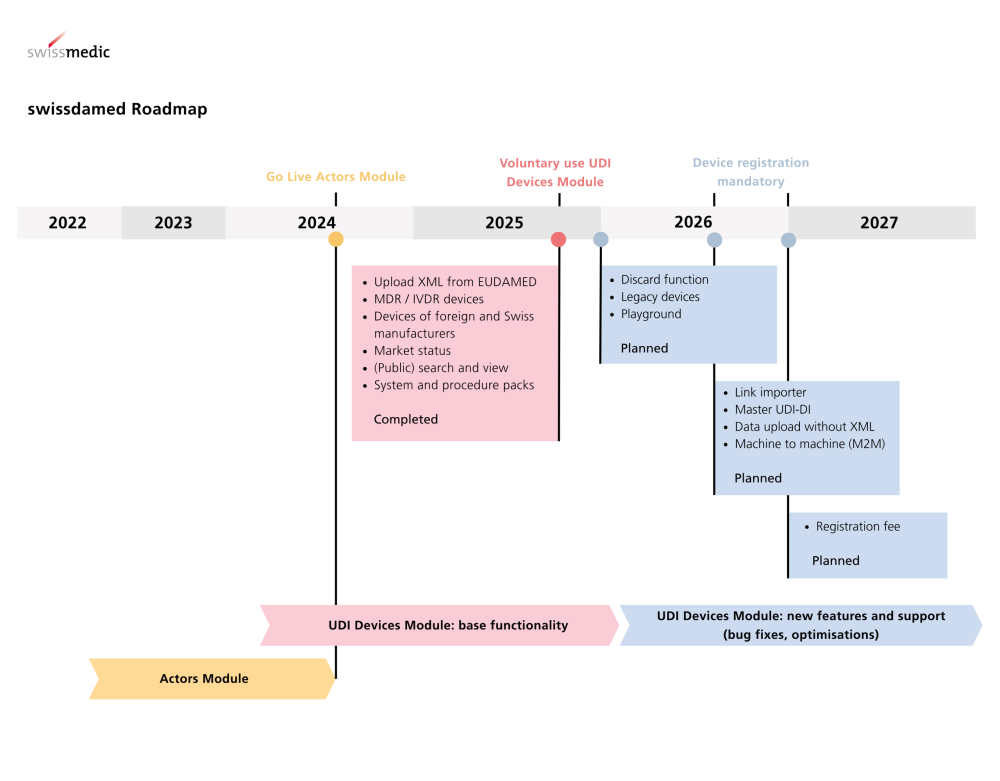
UDI Devices Module Live Swissmedic is expanding the national medical device database with a central functionality. The UDI Devices module in swissdamed has been live since 18 August 2025. From…

Europe IT Consulting Successfully Recertified to ISO 9001:2015 On August 6, 2025, Europe IT Consulting GmbH underwent its surveillance audit conducted by QS Zürich AG. The audit report confirms full…
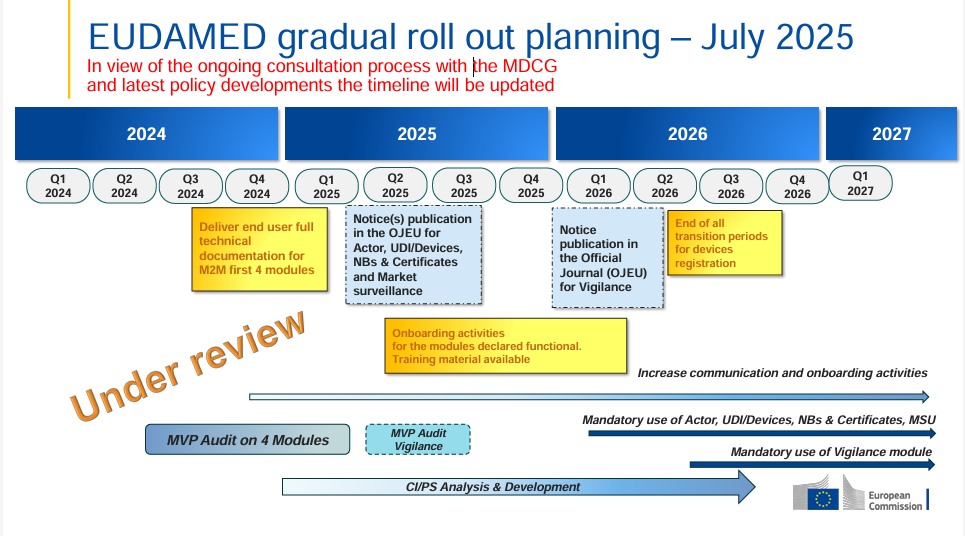
The European Commission has released an updated EUDAMED rollout plan (July 2025) – this time with a clear note: the timeline is now “under review”. This indicates that, as part…
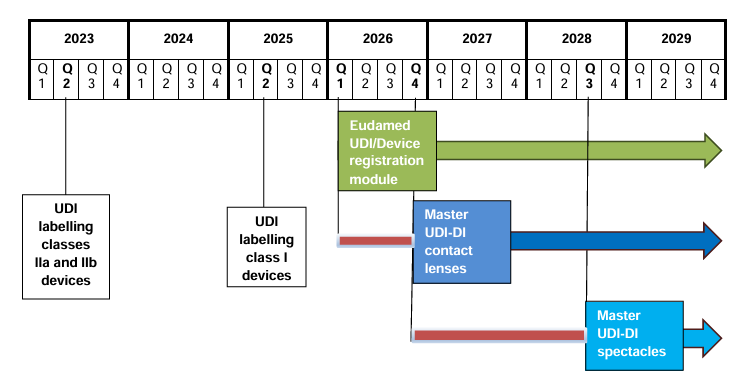
MDCG Publishes Timelines for the Introduction of the Master UDI-DI for Eyewear and Contact Lenses The Medical Device Coordination Group (MDCG) has released its MDCG 2025-7 position paper, setting out…