UDI Devices Module Live
Swissmedic is expanding the national medical device database with a central functionality.
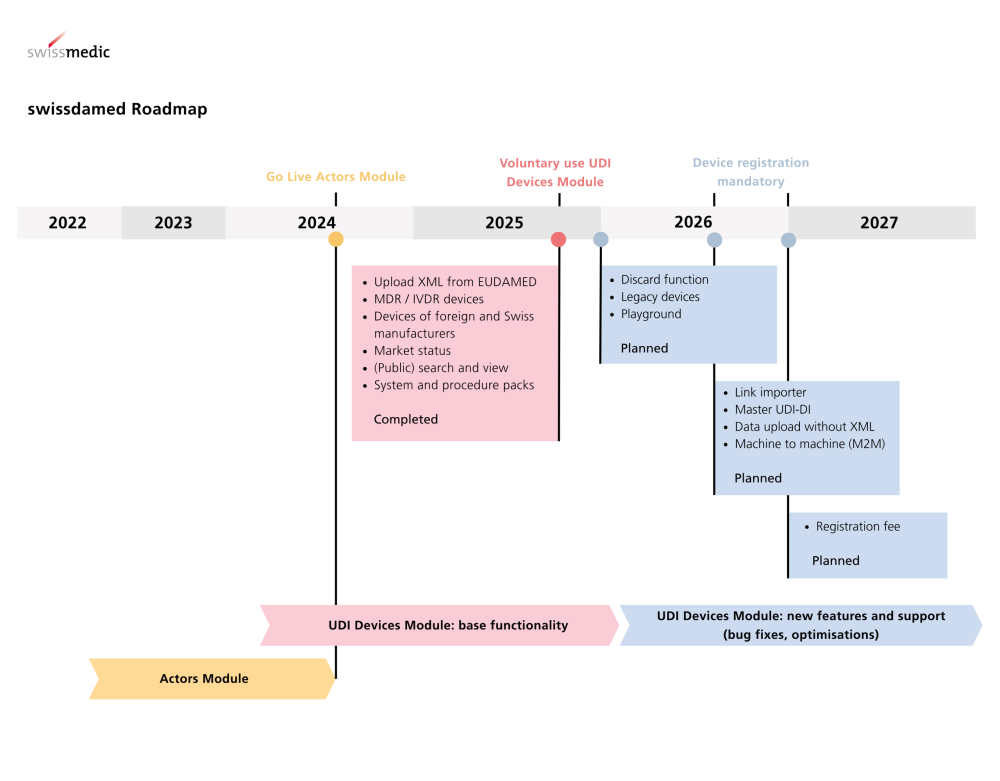
The UDI Devices module in swissdamed has been live since 18 August 2025. From now on , medical devices, in vitro diagnostics as well as systems and process packs can be registered. Registration is initially voluntary, but will become mandatory from 1 July 2026 ; a transitional period until the end of 2026applies to most products.
There is no transitional period for products with a reporting obligation(serious incidents, FSCA, trends ) – the registration obligation will apply from 1 July 2026.
Executive Summary
-
New: UDI Devices module enables product registration in swissdamed.
-
Mandatory: Registration from 01.07.2026, usually with transitional period until 31.12.2026.
-
No transition period: For reportable products (serious incidents, FSCA, trends).
-
Background: swissdamed has been in operation since August 2024 ; Actors module has been active since the start.
What’s new?
The new module complements swissdamed with the registration and management of medical devices, IVDs and systems/process packs – a significant gain in transparency for the Swiss market.
Important dates & transitional arrangements
-
From now on: Registration possible voluntarily .
-
From 1 July 2026: Registration mandatory for products placed on the market in Switzerland. Transition period until 31 December 2026.
-
No transitional period for products with mandatory reporting (serious incidents, FSCA, trends) – mandatory from 1 July 2026.
Economic operators concerned
Registration obligations include Swiss manufacturers, authorised representatives and compilers of system/procedure packs.
Significance for the market
Market transparencygrows with product registration; the step-by-step roll-out strategy allows actors to set up processes in time (actors module since 08/2024, now UDI devices).
What should you do now? (practical)
-
Check actor data: keep the actors module complete & up to date (CHRN, user rights, mandates).
-
Prioritize portfolio: Identify all products/IVDs/systems placed on the market in CH; schedule reportable products first .
-
Ensure data quality: Define UDI master data, responsibilities, change processes and evidence (e.g. FSCA/trend references).
-
Technical preparation: deciding between XML upload (today the way) and later scaling options (templates/automation).
-
Fix schedule: Internal milestones until 01.07.2026 (pilot uploads, reviews, releases).
More information
All details on obligations, schedules, technical basics and other functionalities can be found in the Swissmedic documentation; swissdamed can be reached here: swissdamed.ch.
Leverage our expertise for your successful start with swissdamed.









Related Posts