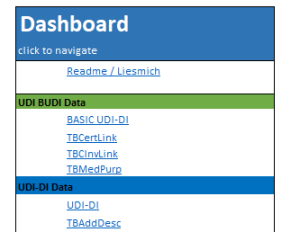
The smart solution for compliant UDI data management
Transform regulatory challenges into competitive advantages
Your problem is our solution
At a time of increasing regulatory requirements, medical device manufacturers face a crucial challenge: How can you efficiently meet the complex EUDAMED requirements without wasting valuable resources?
Our Excel template is the first step – developed by UDI experts for companies that value efficiency and 100% compliance.
Please fill out the form for a quote
Take a look at our powerful Excel template /
Practical demonstration: How easy it is to work with our Excel template
The EUDAMED MDR/IVDR Excel template at a glance:
Comprehensive registers for all required UDI data:
- Basic UDI-DI tab:
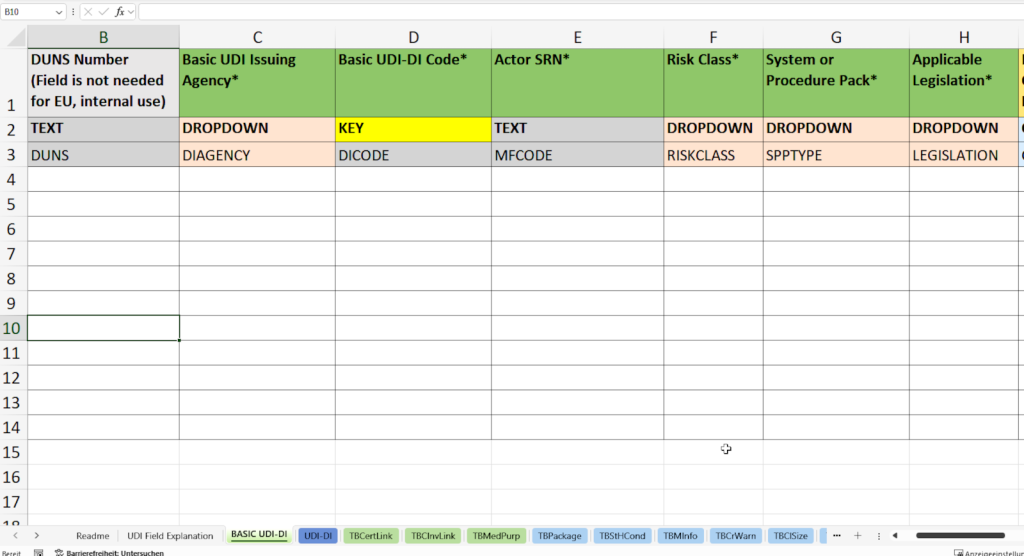 In this tab, you enter all necessary master data for the unique identification of your product family in accordance with the specifications of the EUDAMED database (European Database on Medical Devices).
In this tab, you enter all necessary master data for the unique identification of your product family in accordance with the specifications of the EUDAMED database (European Database on Medical Devices).
- Allocation and documentation of the Basic UDI-DI (Basic UDI-DI Code) according to the specifications of the responsible issuing agency (Basic UDI Issuing Agency), e.g. GS1, HIBCC or ICCBBA.
- Recording the SRN (Single Registration Number, Actor SRN) of your company or the EU Authorized Representative SRN.
- Assignment of the risk class such as class I, IIa, IIb or III according to the MDR/IVDR specifications.
- Selection of product type (System or Procedure Pack)
- Determination of the applicable legislation (Applicable Legislation) – Medical Device Regulation (MDR) or In Vitro Diagnostics Regulation (IVDR).
- Specification of IVDR-specific properties, such as: Companion Diagnostic; Presence of cells/substances of microbial origin; Near-patient testing; Self-patient testing; Use as instrument, kit or for professional testing
- Identification as an active device (Active device), with measuring function (Measuring function) or administration function (Administer device).
- Indication of whether it is a reusable surgical instrument or an implantable device.
- Specification of special device type
- Recording of the device model and/or device name.
- Indication of the use of animal tissues or cells or human components (presence of human tissues or cells).
Please fill out the form for a quote
- UDI-DI tab:
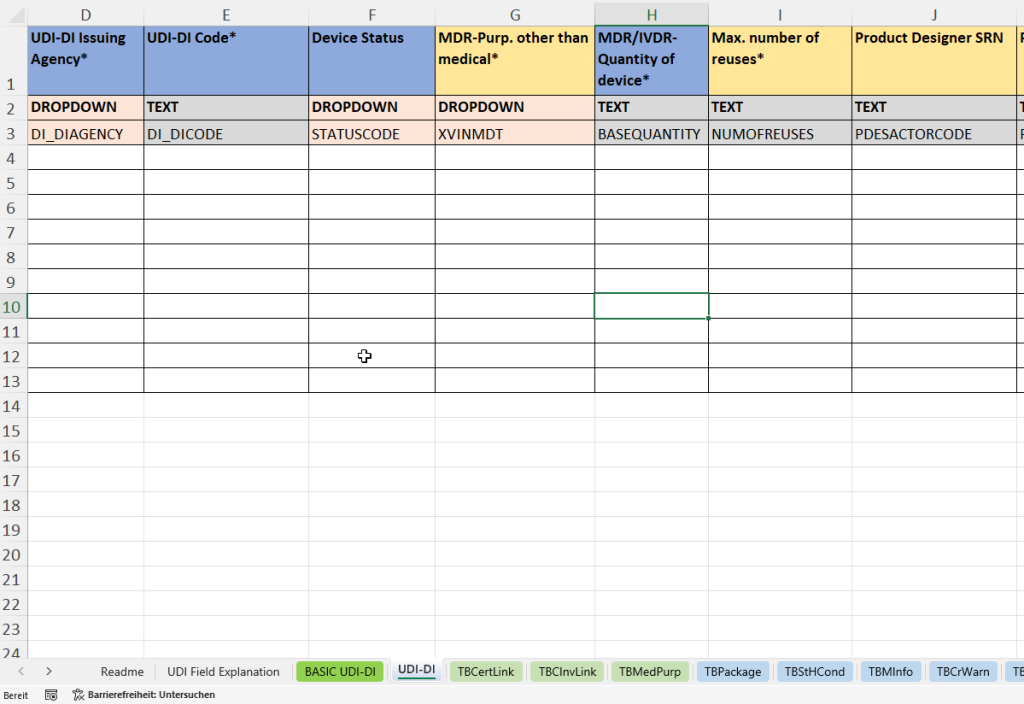 This tab collects all product-related individual data required for the unique identification and traceability of a specific medical device. The UDI-DI (Unique Device Identification – Device Identifier) is the primary key for product registration in EUDAMED.
This tab collects all product-related individual data required for the unique identification and traceability of a specific medical device. The UDI-DI (Unique Device Identification – Device Identifier) is the primary key for product registration in EUDAMED.
- Linkage with the parent basic UDI-DI (Parent Basic UDI DI Code) for classification in a product family.
- Entry of the material number (material) and material description (material text) for internal reference.
- Award of the UDI-DI by an accredited agency (UDI-DI Issuing Agency) and entry of the unique code (UDI-DI Code).
- Determination of the device status (Device Status)
- Indication of the purpose other than medical (MDR Purpose other than medical)
- Recording of the number of pieces per sales unit (quantity of device) and the maximum reusability (max. number of reuses).
- Specification of the product developer (Product Designer SRN and Product Designer ID).
- Unique reference number for identification in documents and packaging.
- Direct marking on the product (direct marking) and information on: same DI for direct marking as for UDI-DI (DM DI same as UDI-DI code); issuing agency for direct marking (DM issuing agency); direct marking code (DM DI code)
- Sterile labelling (Device labelled as sterile) and sterilisation requirement before use (Device needs sterilisation before use)
- Indication of whether it is a new product (new device) according to IVDR
- Contains latex (Containing latex)
- Reprocessed single-use device
- Labelled as single use
- Production Identifier (PI): Lot or Batch number; Expiration Date; Manufacturing Date; Serial Number and Software Identification
- Additional identifiers: Secondary Issuing Agency (UDI) and Secondary UDI-DI Code (UDI-DI Code) and Unit of Use DI Code (UDI) and associated Issuing Agency (Unit of Use Issuing Agency)
- Further information: URL for additional information (eIFU)
- Certificate Information:
- Entry of the associated Basic UDI-DI code (Basic UDI Code) for unique linking to the certificate.
- Entry of the certificate number (Certificate No.) according to the Notified Body (NB) and management of the revision number (Certificate Revision No. – Cert. rev. no.), e.g. in case of renewal or change.
- Selection of the certificate type (Certificate Type – Cert. type) from a predefined list (e.g. EU certificate of conformity, QMS certificate) and entry of the Notified Body Code (NB Code – Notified Body Code), as well as indication of the expiry date of the certificate if it is a product under MDD, IVDD or AIMDD.
- Clinical Investigation
- Registration of the Clinical Investigation ID according to the study protocol or database entry.
- Indication of the country in which the clinical trial was conducted
- Language & Medical Purpose
- Selection of the language in which product-related information is provided (e.g. label, instructions for use, EUDAMED data).
- Indication of medical purpose
- Packaging information (Packaging Hierarchy):
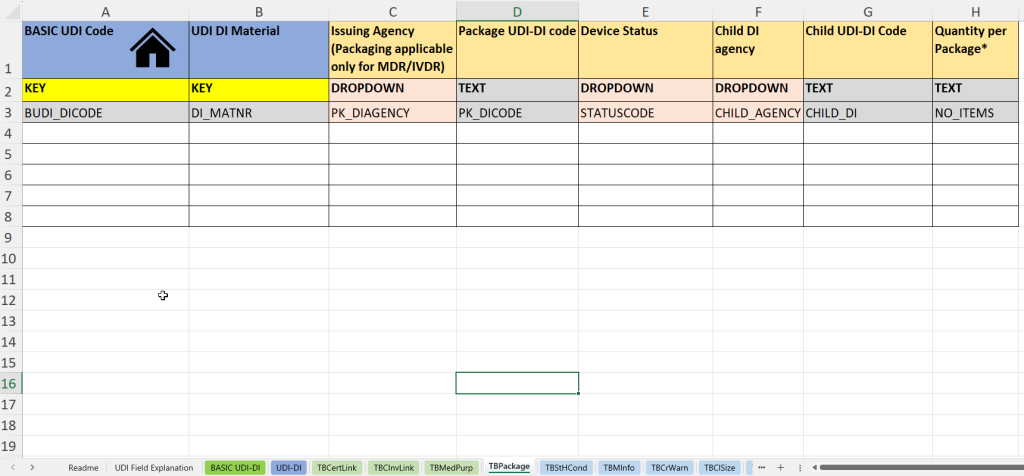 This tab documents information on packaging units as well as on child UDI-DI structures (“Child UDI”) – particularly relevant for multi-level packaging or sets.
This tab documents information on packaging units as well as on child UDI-DI structures (“Child UDI”) – particularly relevant for multi-level packaging or sets.
- UDI-DI material number (UDI DI material) for internal identification of the packaging unit.
- Issuing agency for packaging UDI-DI (Issuing Agency – Packaging)
- Entry of the UDI-DI code of the packaging (Package UDI-DI Code) – e.g. for outer packaging or kits.
- Status of the product or packaging (device status) – e.g. active/inactive.
- Indication of the Child UDI issuing agency (Child DI Agency), if it concerns subordinate UDI-DIs.
- Child UDI-DI Code: UDI code of the child product within the packaging unit.
- Quantity per package – important for sets, blisters or multipacks.
- Storage & Handling Conditions:
-
UDI-DI material number (UDI DI material) to identify the unit in question.
-
Specification of storage and handling conditions (Storage & Handling Conditions Type).
-
Language for the description and supplementary description for a more detailed explanation of special storage requirements or notes.
-
Please fill out the form for a quote
- Market information:
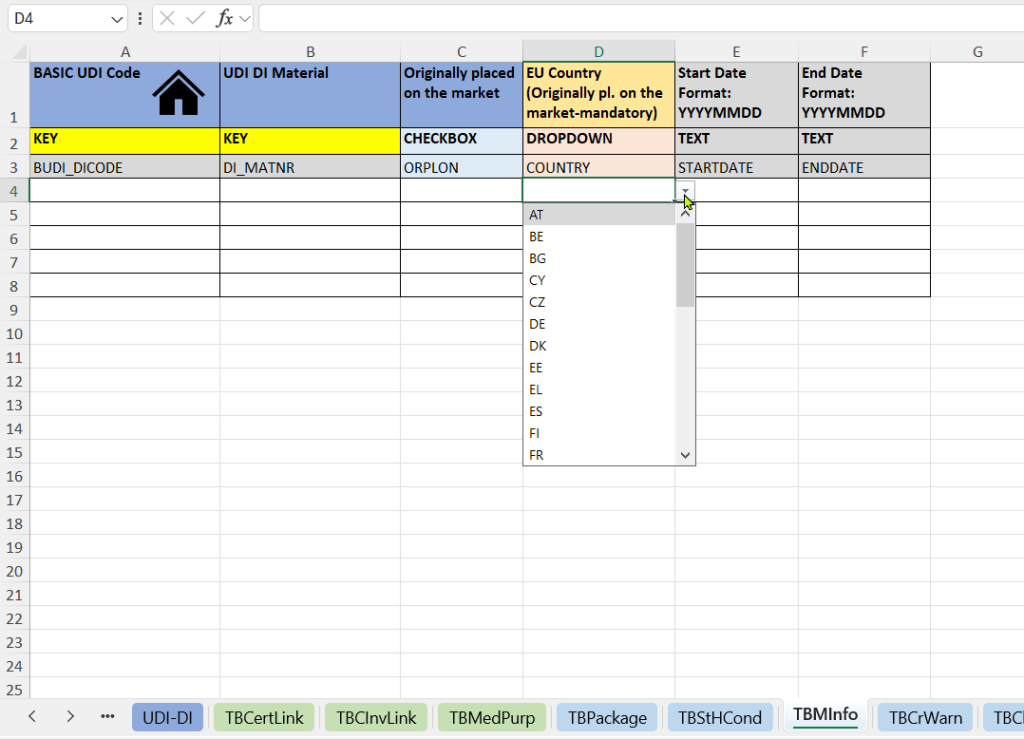 This tab documents when and where a product was first placed on the market. It provides regulatory-relevant evidence for MDR/IVDR-compliant products and legacy products.
This tab documents when and where a product was first placed on the market. It provides regulatory-relevant evidence for MDR/IVDR-compliant products and legacy products.
- Indication of whether the product was originally placed on the market.
- EU Country – Originally placed on the market.
- Market launch date (Start Date and End Date).
- Critical Warning
- Selection of the Critical Warning Type – e.g. implant, latex, radioactivity, etc.
- Size Information
- Specification of the size format (size type) – e.g. numeric, alphanumeric, symbolic
- Accuracy/precision of the size specification (precision)
- Size Unit
- Value or minimum value (Value / Min. value) and maximum value (Max Value)
- Human Substances (Medical Human Substance Tab)
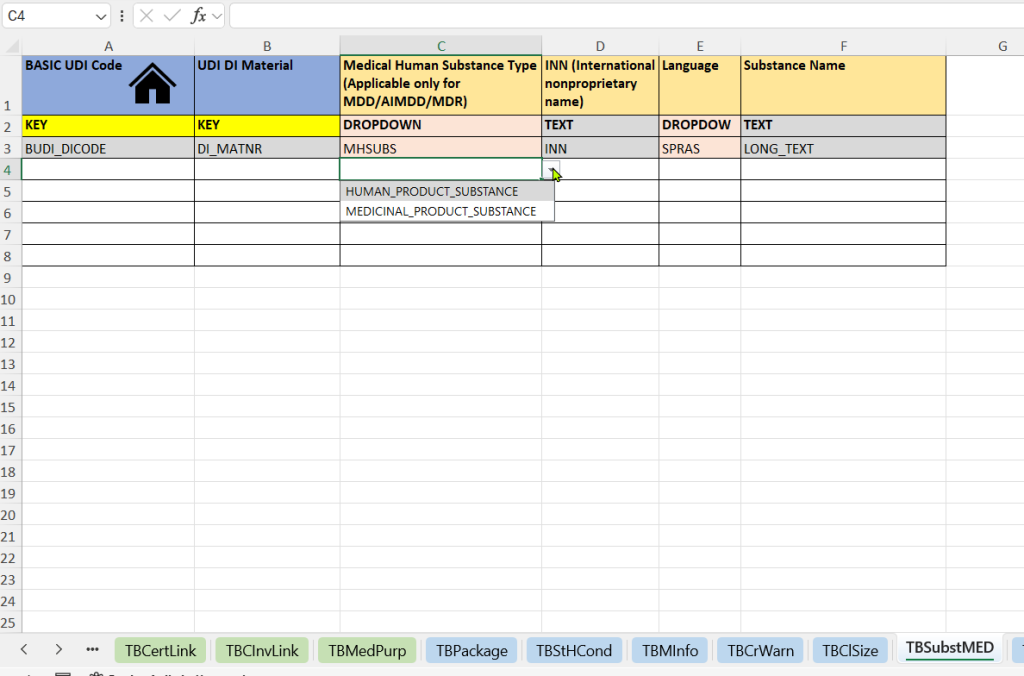
-
Selection of the substance type (Medical Human Substance Type)
-
Registration of the International Nonproprietary Name (INN)
-
Specification of the language – for the language-specific representation of the substance specification
-
Free text name of the substance (Substance Name) – additional description or nationally used name
-
- CMR/Endocrine Substances (CMR Endocrine Substances)
-
Selection of substance type (CMR Endocrine Substances) – e.g. CMR category 1A/1B, endocrine disruptors (field: CMRENDSUBS).
-
Specification of the CAS code – Chemical Abstracts Service Identifier (field: CASCODE).
-
Specification of the EC/Einecs/EC code (EC code) – European substance identifier (field: ECCODE).
-
Language – for localized descriptions, if required (field: SPRAS).
-
Free text name of the substance (substance name) – e.g. commercial name or specific description (field: LONG_TEXT).
-
- Additional Product Description
-
Selection of the language in which the additional description is to be made (field: SPRAS).
-
Recording of the additional product description
(field: DESCR – e.g. to explain the function, composition or special feature of the set)
-
- Trade Name Tab
-
Selection of the language for the trade name (field: SPRAS).
-
Trade Name Registration
(Required – Field: TRD_NAME, e.g. “CardioFlow Stent”, “MediCheck Rapid Test Kit”)
-
- Nomenclature code (EMDN Nomenclature)
- Selection of the appropriate EMDN code (Nomenclature Code – EMDN) from the official EUDAMED database.
Please fill out the form for a quote
Smart features for maximum efficiency:
- Colour highlighting of mandatory fields:
- Immediate visual identification of mandatory entries
- Distinction between conditional and absolute mandatory fields
- Contextual display of relevant fields based on previous inputs
- Validated drop-down menus:
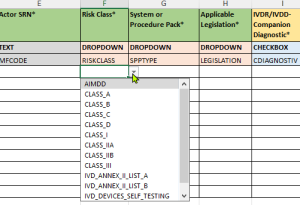
- Pre-defined choices according to EUDAMED requirements
- Updated value lists according to the latest EU Commission specifications
- Reduction of input errors through standardized options
- Automatic dependency checks:
- Dynamic adjustment of input fields based on product type and classification
- Logical check of data consistency between different tabs
- Warnings for potential inconsistencies in data entry
- Bilingual support:
- Parallel descriptions in German and English
- International usability for global teams
- Consistent terminology according to official EUDAMED documentation
- Documentation aids:
- Integrated explanations of complex fields
- Notes on regulatory requirements
- Tips for optimal data entry
Comprehensive range of services for your EUDAMED compliance
- EUDAMED MDR/IVDR Excel template: the professional basis for your structured UDI data management with all the functions described above.
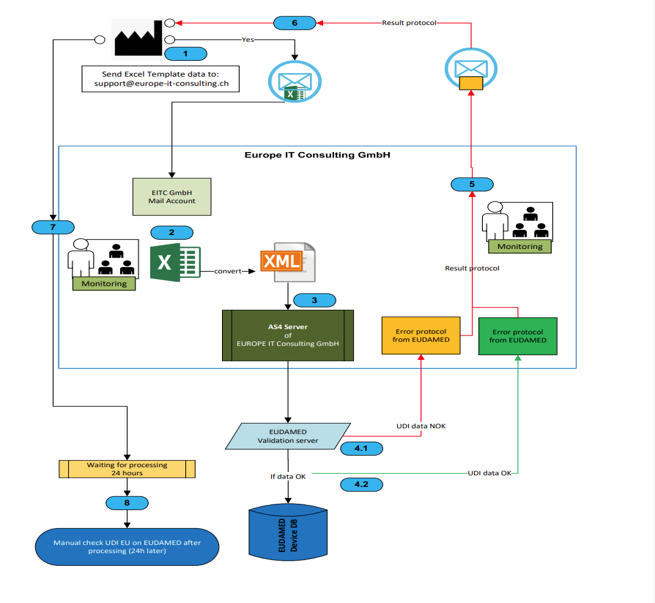
- XML conversion service: Important: The conversion of the Excel data into the XML format required by EUDAMED does not take place automatically within the template. Our experts take over this complex task for you as a separate service!
- EUDAMED Transmission Service: We take care of the entire process of transferring data to the EUDAMED database – securely, quickly and reliably.
Excel Template Technical Specifications
- File format: Microsoft Excel (.xlsx)
- Compatibility: Microsoft Excel 2016 and higher
- Size: Optimized for fast loading times, even with large data sets
- Security: Password-protected structures to prevent unintentional changes
- Updates: Regular updates according to the latest EUDAMED requirements
- Languages: German and English
- Data validation: Integrated check routines for consistent inputs
Please fill out the form for a quote
Why leading medical technology companies rely on our solution
Europe IT Consulting GmbH is not only ISO 9001 certified and SAP partner – we are your strategic partner for the digital transformation of your regulatory processes.
This is what makes our Excel template unique:
- Precise structuring according to EUDAMED requirements for basic UDI-DI and UDI-DI attributes
- Smart drop-down menus with validated choices
- Visual highlighting of mandatory fields and dependent fields
- Intuitive navigation through the complex world of UDI data
- Multilingual support for international teams (DE/EN)
- Optimal preparation for further processing by our experts
- ANALYZE: Recognize the value of our total solution for your specific challenges. “The first step to compliance optimization”
- PURCHASE: Get easy access to our Excel template via Digistore24. “The cornerstone of your regulatory strategy”
- IMPLEMENT: Capture your product data in the user-friendly template structure. “No more complicated UDI data entry”
- BENEFIT: commission us with XML conversion and EUDAMED transfer – and enjoy a fully secured EUDAMED workflow. “Focus on your core business while we take care of the technical implementation”
Perfectly tailored to your individual requirements
For small and medium product portfolios:
The Excel template is the ideal basis for data collection for up to 20 records – supplemented by our conversion and transfer service.
For extensive product lines:
The perfect basis for M2M integrations with larger amounts of data (over 20 UDI-DI entries) – with additional support from our IT experts.
For SAP users:
Maximize your benefits through seamless integration with our UDI EUDAMED SAP Add-On.
What our customers say
“The combination of Europe IT Consulting’s Excel template and conversion service has revolutionized our EUDAMED submission process. What used to take days, we now do in hours – with significantly higher data quality.” — Medical technology manufacturer, Germany
“The intuitive structure of the Excel template and the professional support in XML conversion and EUDAMED transfer give us the security of always staying compliant.” — Regulatory Affairs Manager, Switzerland
“Particularly valuable are the predefined drop-down menus and the colour marking of the mandatory fields. This way, we always have an overview of which data is absolutely necessary.” — Quality Manager, Austria
Frequently asked questions to make your decision easier
Are there any additional costs for updates to the Excel template?No, all important updates to the template are included in your purchase at no extra charge.
Can I create XML files for EUDAMED myself with the Excel template?No, the Excel template is used exclusively for the structured collection of your UDI data. We offer the conversion to the complex EUDAMED XML format as a separate service, as this process requires special technical expertise.
How is my data transferred to the EUDAMED database?After collecting data in the Excel template, you can use our transfer service. Our experts convert your data into the correct format and transmit it securely to the EUDAMED database.
How is my entered data backed up?The Excel template has built-in functions for local data storage. We also recommend regular backups according to your company’s internal security guidelines.
Can I use the template for multiple products or product lines?Absolutely. The template is designed to manage multiple Basic UDI-DIs and numerous associated UDI-DIs in a clear structure.
Do I need special Excel knowledge to use the template?No, the template was developed for users with basic Excel knowledge. The intuitive user interface and clear structuring make it easy to enter even without special prior knowledge.
Will the template be updated in case of regulatory changes?Absolutely! We proactively update all value lists and integrate new requirements as soon as the European Commission publishes them – your guarantee of ongoing compliance.
Act now – secure your regulatory edge!
Time is running out and the EUDAMED deadlines are approaching. Choose the complete solution that leading medical technology companies trust today.
Your first step to optimized UDI compliance:
Please fill out the form for a quote
Invest in an efficient regulatory strategy now!
The EUDAMED MDR/IVDR Excel template is developed and distributed by Europe IT Consulting GmbH, your certified partner for regulatory IT solutions since 2011.
