We provide comprehensive advice on all matters regarding UDI FDA/EUDAMED. With our experience from already realized projects, we can provide you with the best answers.

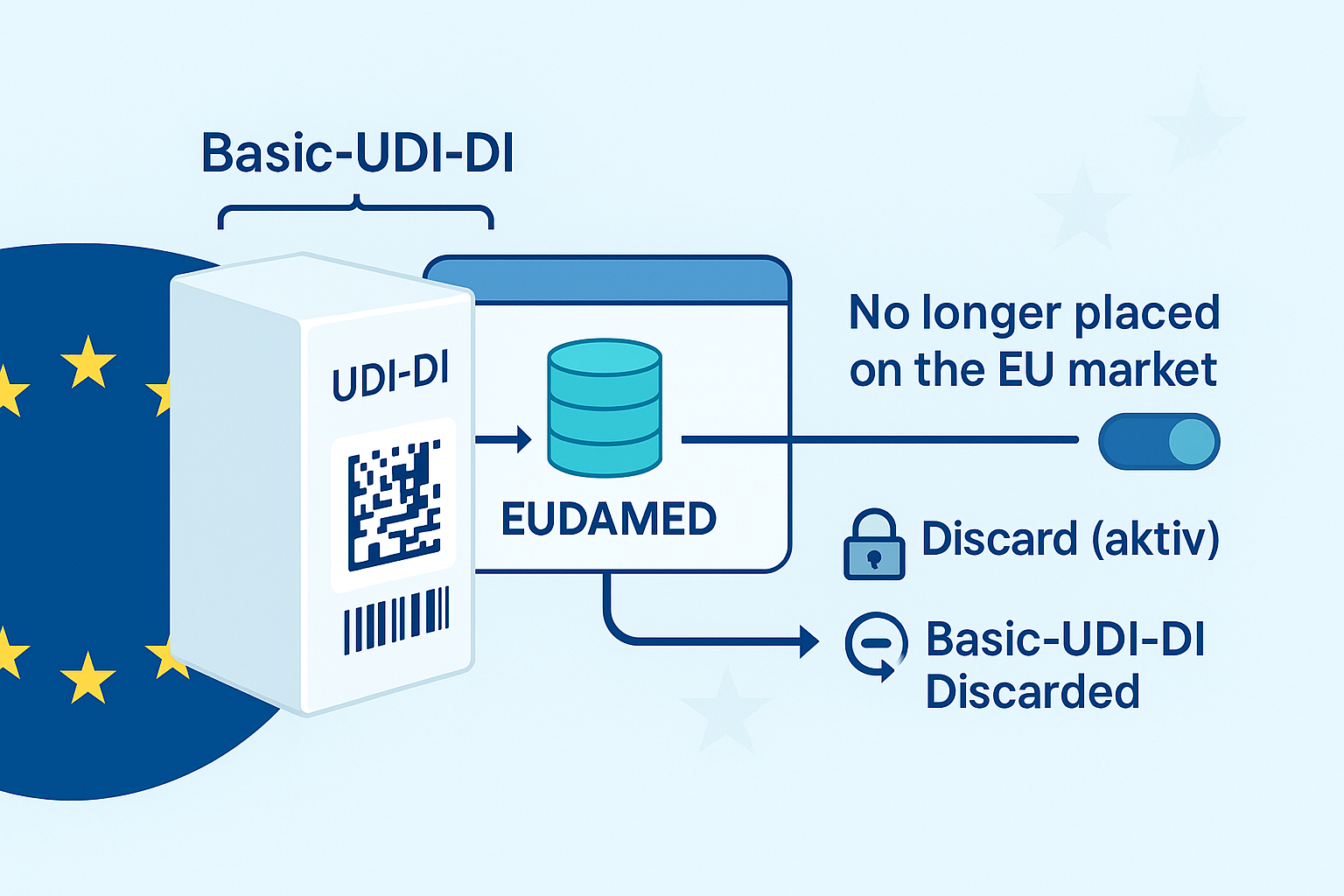
UDI implementation in SAP
In order to meet the legal requirements of the FDA, manufacturers of medical technology products will have to comply with the UDI (Unique Device Identification) requirements in the future.