Basic UDI-DI in transition scenarios: Can a BUDI be "empty"?
1. Can a Basic UDI-DI (transitional) exist without associated UDI-DI(s)?
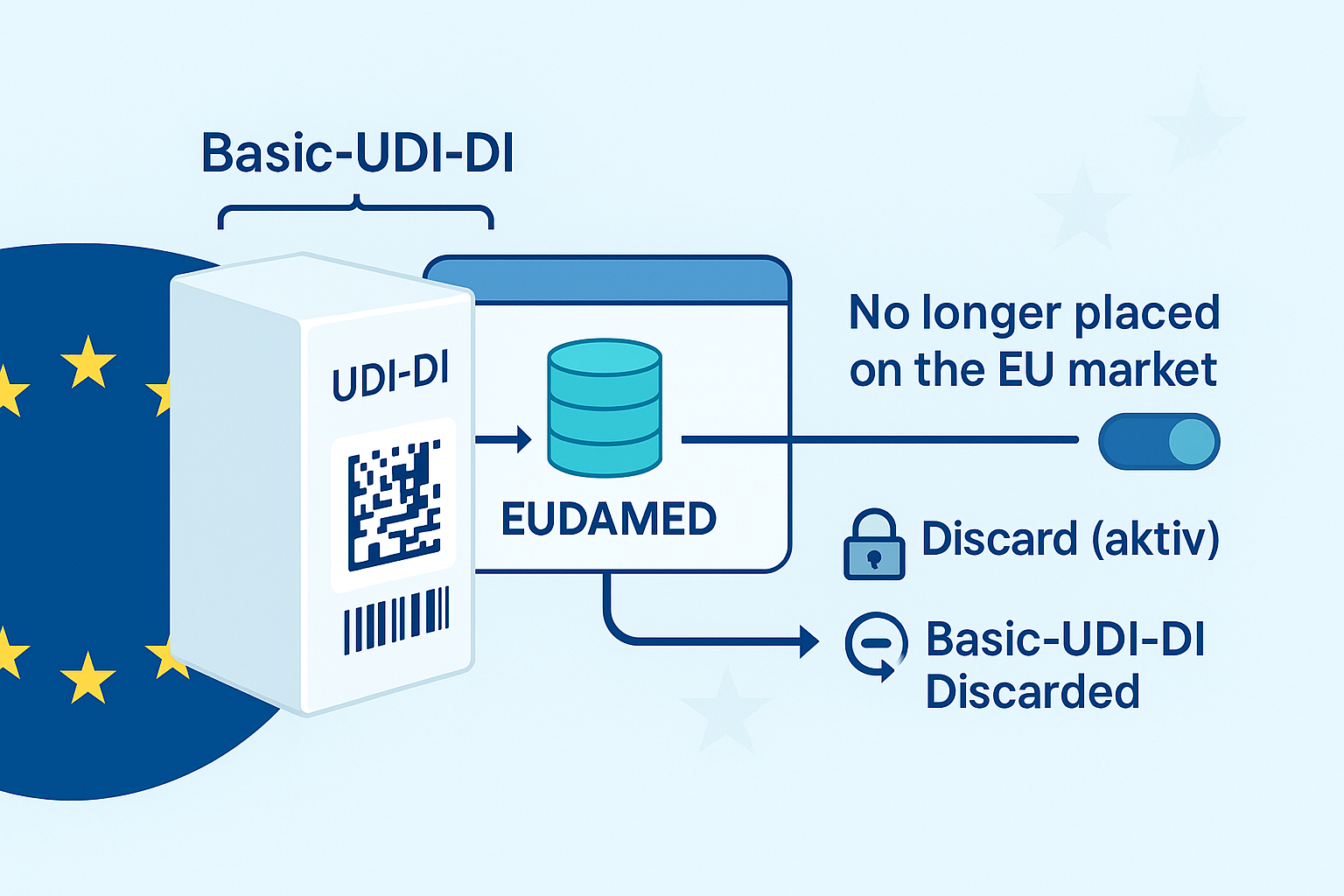
No – in EUDAMED, a basic UDI-DI is not registered in isolation. When registering Regulation Devices , the official EUDAMED user guide requires that a Basic UDI-DI is always submitted together with at least one UDI-DI ("…you cannot register a Basic UDI without a UDI-DI"). European Commission – UDI Devices User Guide
2. What happens to the Basic UDI-DI if the last associated UDI-DI is set to "No longer placed on the EU market"?
The status change does not delete either the UDI-DI or the Basic UDI-DI. According to the user guide, the status "No longer placed on the EU market" hides the market information; linked container packages are automatically set to the same status. Deletion is not automatic, but only by "Discard" (conscious action). European Commission – UDI Devices User Guide
However, if you actively "discard" the last UDI-DI, according to official business rules: then the associated Basic UDI-DI is also set to "Discarded" (logical delete); "Discarded"entries are no longer publicly visible and codes can be reused. In addition, "Discard" is blocked if, for example, the Basic UDI-DI is referenced in certificates or the device occurs in vigilance messages. European Commission-Business Rules
3. What if the product was not registered in EUDAMED at all, but a BUDI already exists internally?
-
On the EUDAMED side, no data record exists – since a basic UDI-DI cannot be registered without UDI-DI (see 1).
-
Legal (MDR): Before placing on the market, the manufacturer must submit the Basic UDI-DI including core data to the UDI database; the Basic UDI-DI is the master key in the UDI database and is mentioned in certificates and in the EU declaration of conformity (Annex VI/IV). EUR-Lex+1
-
On the current obligation to use EUDAMED: The use of the UDI/device module is currently (as of today) not yet binding according to the EU Commission; entries are voluntary until the respective modules become mandatory by official journal notification (phase-by-phase roll-out; new legal situation 2024/1860). National additional obligations are possible.









Related Posts