regularia 2026 Video

regularia 2026 on March 3rd in the Tuttlingen town hall

regularia 2026 on March 3rd in the Tuttlingen town hall

Merry Christmas and a great start to 2026! An intense year is drawing to a close – with many projects around UDI, EUDAMED, FDA GUDID & more. Together with you,…
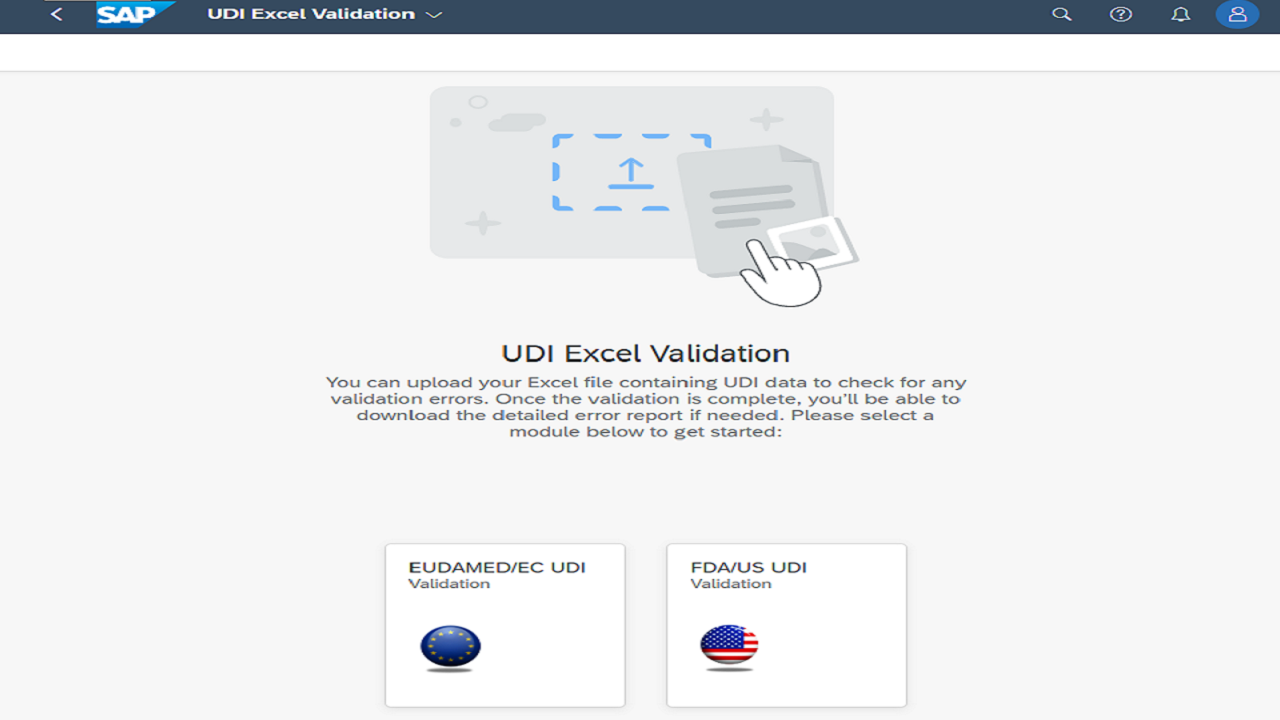
UDI Excel Cloud Validator from Europe IT Consulting GmbH Now Available on SAP® Store By integrating with SAP Business Technology Platform, the UDI Excel Cloud Validator from Europe IT Consulting…
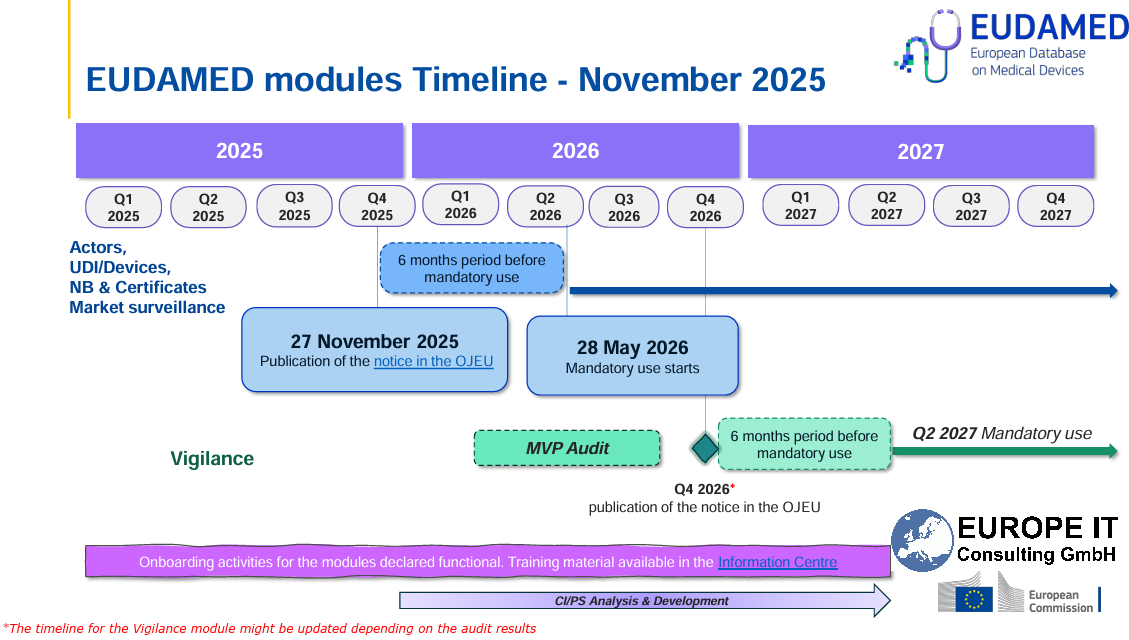
EUDAMED: Four Modules Mandatory from 28 May 2026 With the publication of a new Commission Implementing Decision in the Official Journal of the European Union on 27 November 2025, the…
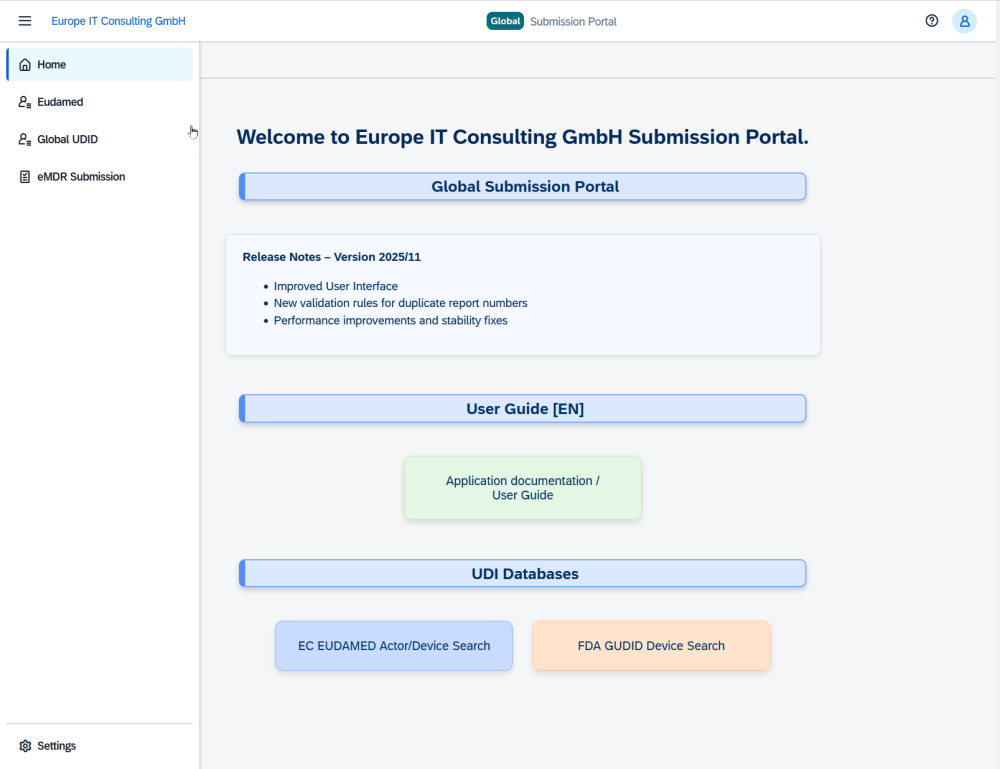
Europe IT Consulting introduces the Global Submission Portal with a focus on FDA eMDR New cloud platform combines eMDR, GUDID and EUDAMED in a single system With the Global Submission…

Save the date: regularia 2026 – Europe IT Consulting will be there On 3 March 2026, the regularia will take place for the first time at Stadthalle Tuttlingen – the…

What Contact Lens Manufacturers Need to Know About the Master UDI-DI At the end of August 2025, the Medical Device Coordination Group (MDCG) published its revised guidance MDCG 2024-14 Rev.1.…
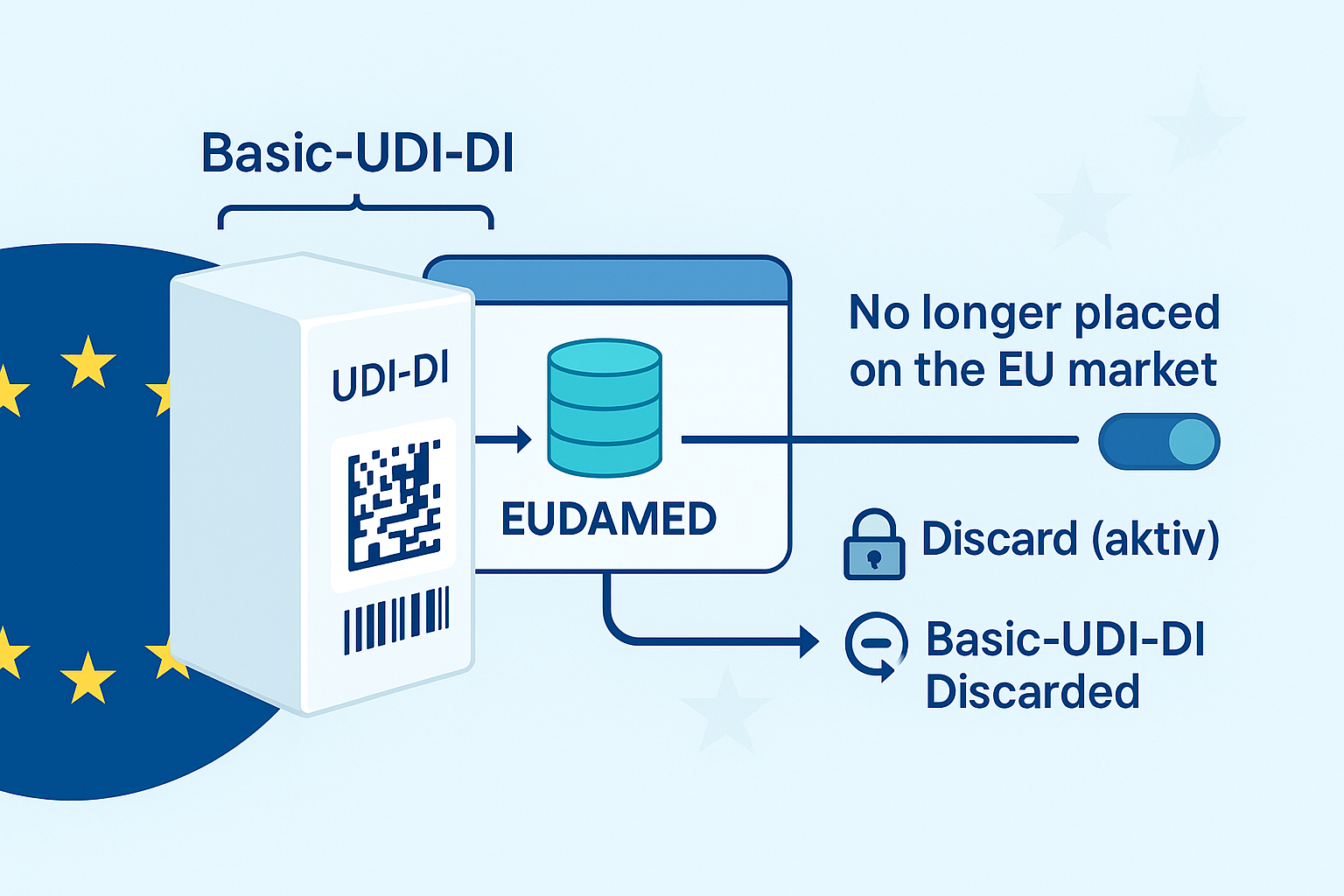
Basic UDI-DI in transition scenarios: Can a BUDI be "empty"? 1. Can a Basic UDI-DI (transitional) exist without associated UDI-DI(s)? No – in EUDAMED, a basic UDI-DI is not registered…

EU Launches "Apply AI": €1 Billion for AI in Key Industries – Focus on Healthcare The European Commission is launching "Apply AI", an investment package worth €1 billion, to accelerate…
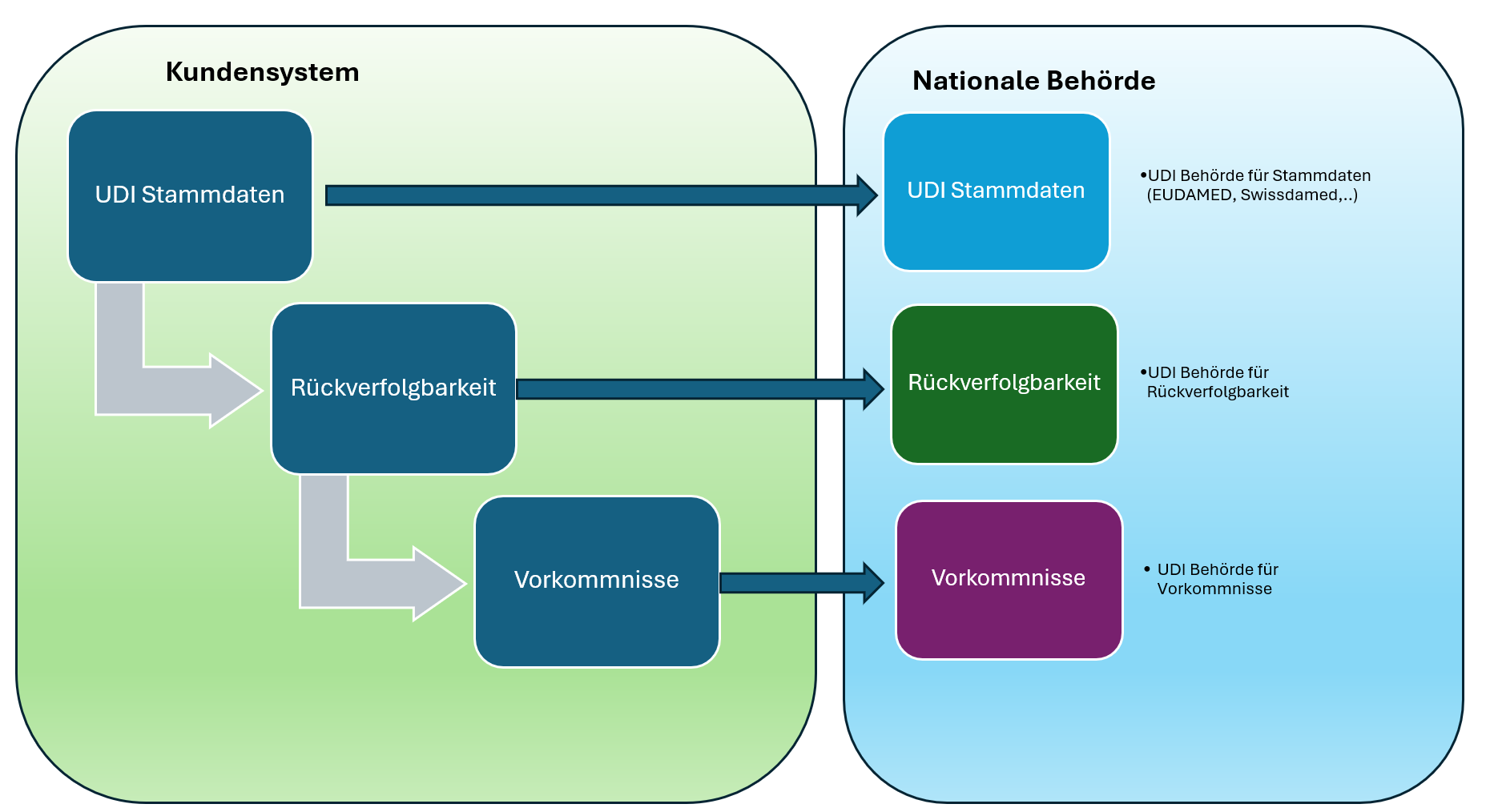
The full UDI lifecycle – everything from a single source The path to full UDI compliance can be understood as a data cycle: UDI master data → Traceability → Vigilance…