UDI Lifecycle
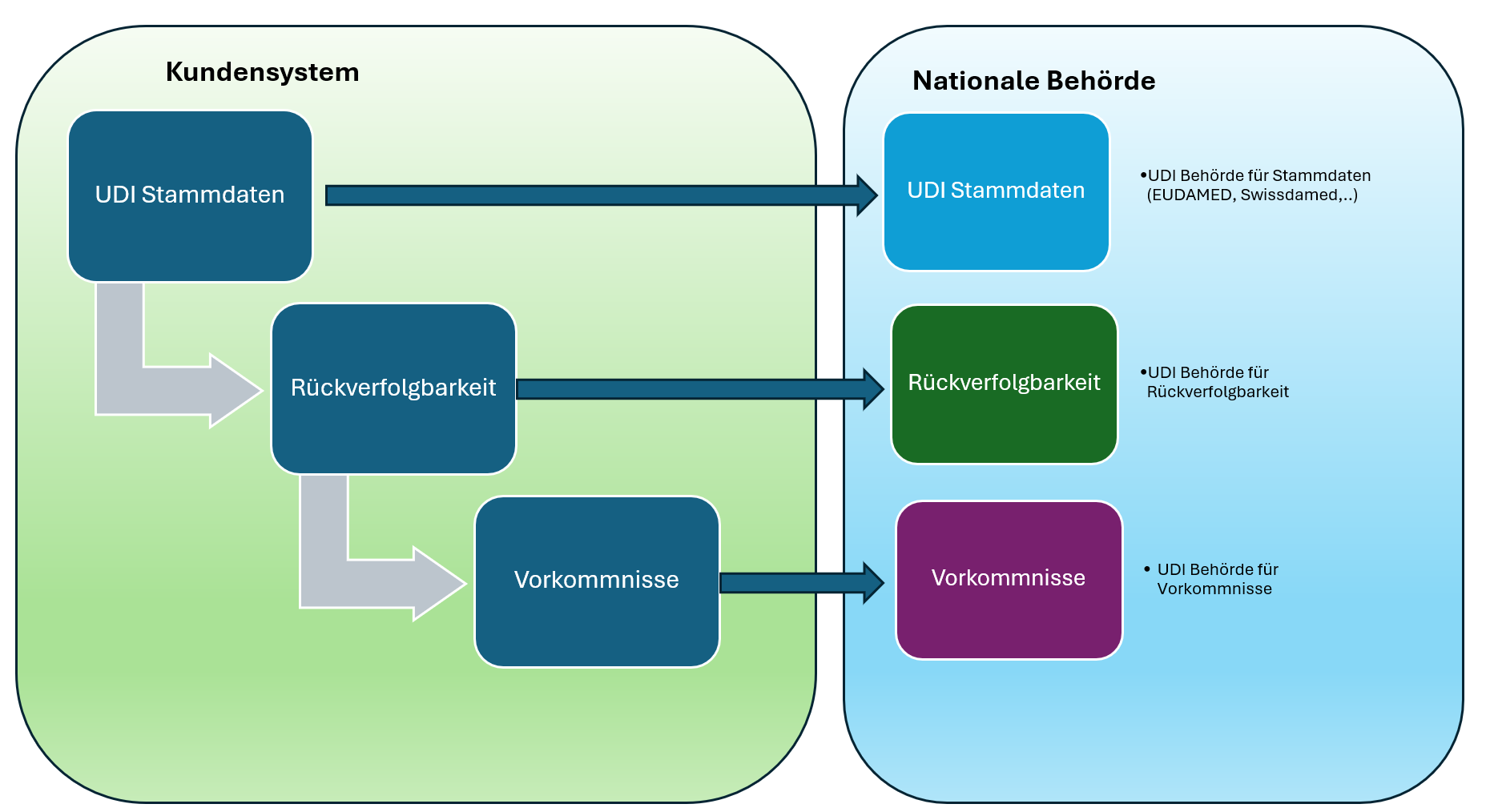
The full UDI lifecycle – everything from a single source The path to full UDI compliance can be understood as a data cycle: UDI master data → Traceability → Vigilance…

The full UDI lifecycle – everything from a single source The path to full UDI compliance can be understood as a data cycle: UDI master data → Traceability → Vigilance…

When does the UDI-DI status need to be changed?In EUDAMED, the status (not the country end data) decides whether a device is considered "On the EU market" or "No longer…
Do custom-made devices (CMD) have to be registered in EUDAMED – and what about cases of vigilance? What are custom-made products according to the MDR? Please also read our article…