
When does the UDI-DI status need to be changed?
In EUDAMED, the status (not the country end data) decides whether a device is considered "On the EU market" or "No longer placed on the EU market". Anyone who terminates the first placing on the market throughout the EU must set the status to "No longer…" – pure end data per country does notchange the status display.
The statuses in EUDAMED
-
On the EU market
-
No longer placed on the EU market
-
Not intended for the EU market (only as initial status, cannot be set via update)
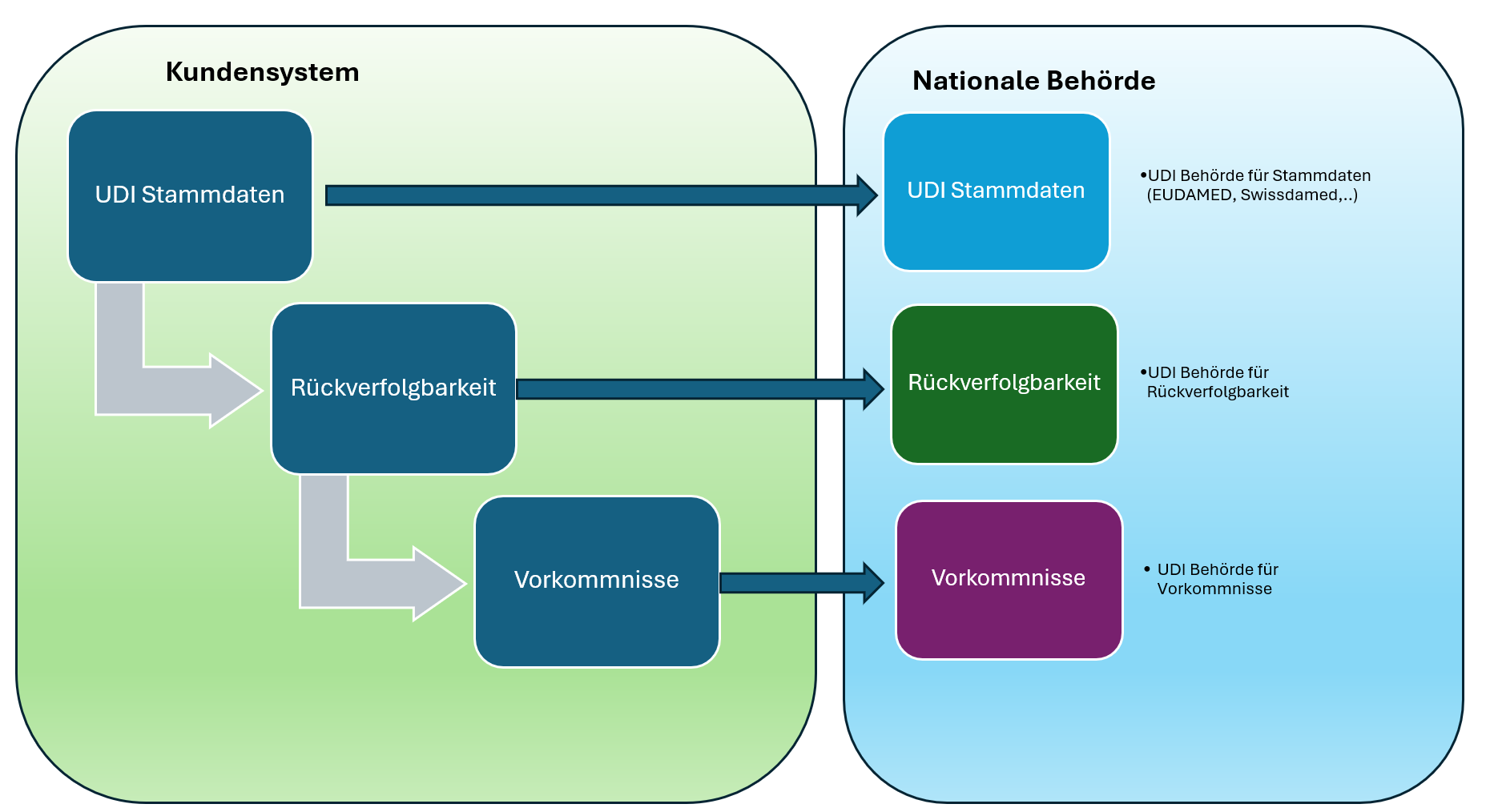
Key Difference: Status vs. Market Information
-
Control countries & end data ("Market information") where the product was/is (still) available. This information alone does not change the status. webgate.ec.europa.eu
-
If the status is "No longer placed on the EU market" or "Not intended for the EU market", market information is no longer applicable (will be hidden in the UI). webgate.ec.europa.eu
-
Updates to market information are versioned (EUDAMED creates versions when changes are made).
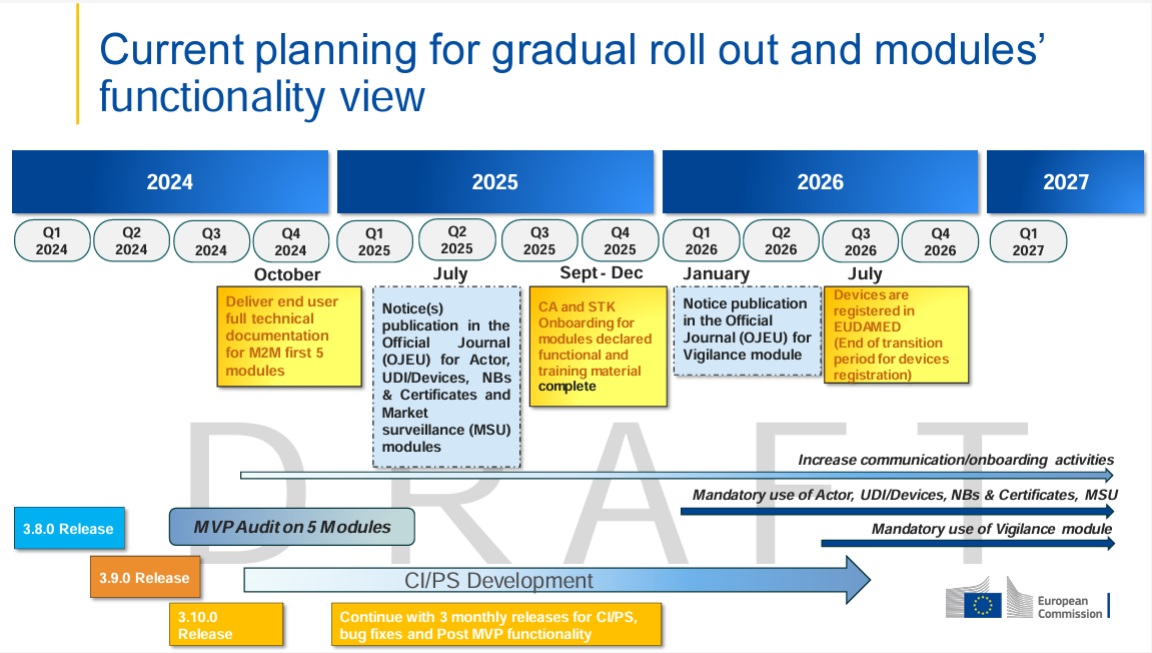
When to set which status?
| Situation | Maintain what? | Correct Status |
|---|---|---|
| You are still selling in at least one EU country | Keep market information per country (start/end) up to date | On the EU market |
| You discontinue "Placing on the market" throughout the EU (no further initial provision by the manufacturer) | Create new version and change status | No longer placed on the EU market |
| A product was never intended for the EU and is re-registered | Set initial status | Not intended for the EU market (only initially possible) |
Effects of "No longer…"
-
Market information is no longer relevant/viewable.
-
Container packages automatically assume the device status: When changing on the EU market → No longer… , EUDAMED automatically switches linked container packages to No longer… .
How to implement it correctly in EUDAMED (short how-to)
-
Select new version of the UDI-DI create/open → status according to the above guide.
-
When switching to "No longer…" , EUDAMED confirms the automatic status transfer for linked container packages.
-
Maintain market information (first country + availability per country) only as long as "On the EU market" applies.
Mini FAQ
-
Are country end data ("valid to date") sufficient?
No. End dates do not change status. For an EU-wide sales stop, you must switch to "No longer placed on the EU market" . -
May the status "No longer…" be set initially?
Yes, that is permissible. -
What happens to container packages when the status changes?
You inherit the status of the device; when changing on the EU market → No longer… , the changeover takes place automatically.
References
- EUDAMED Business Rules & Enumerations (UDI/Devices), incl. Status logic, inheritance to container packages and applicability of market information. webgate.ec.europa.eu
- EUDAMED Release Notes v2.13 (automatic status transfer for container packages; initial status "No longer…" possible). webgate.ec.europa.eu
- MDR/IVDR definitions of "placing on the market"/"making available". EUR-Lex





Related Posts