We would like to inform you today about the latest developments regarding the Vigilance module in the EUDAMED database. This crucial module, scheduled to go live in 2026, will be essential for medical device manufacturers to meet regulatory requirements and ensure product safety.
What is the EUDAMED Vigilance Module?
The Vigilance module of EUDAMED (European Database on Medical Devices) is designed for the recording and reporting of serious safety incidents, adverse events, and field safety corrective actions related to medical devices. It facilitates transparent and efficient communication between manufacturers and authorities regarding safety-related information.
Regulatory Obligations and Compliance
Under the new Medical Device Regulation (MDR), the use of the Vigilance module is mandatory for all medical device manufacturers. Companies must ensure that they report all serious incidents and corrective actions accurately and on time to avoid penalties or restrictions on product distribution.
Structure of the Module
The Vigilance module will allow incidents and actions to be reported electronically to the relevant authorities. It is divided into several sections:
- Reporting serious incidents: Detailed information on incidents that could cause harm to health.
- Field safety corrective actions: Information about corrective actions taken to mitigate risks.
- Reports and feedback: Tools for tracking and communicating status reports.
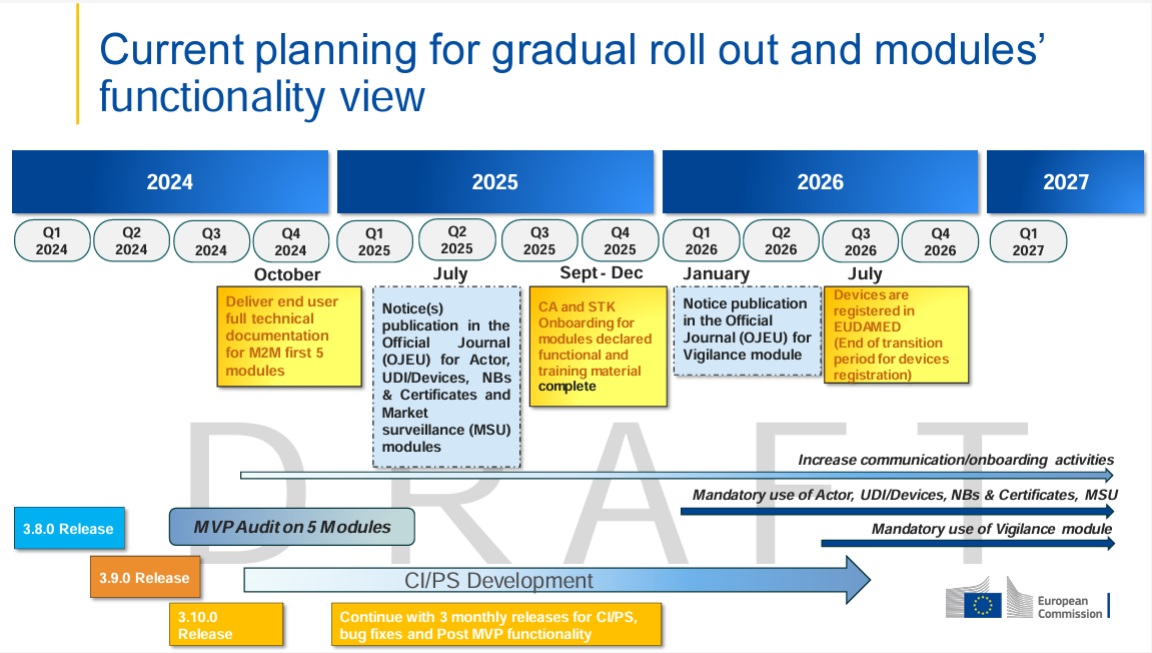
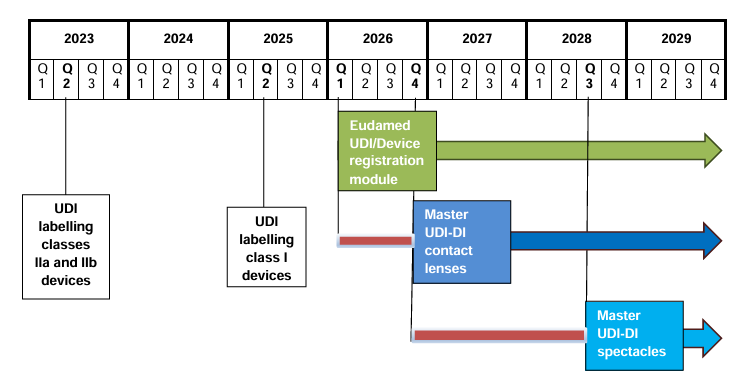
When Will the Vigilance Module Be Active?
The Vigilance module is expected to go live in the first quarter of 2026, with mandatory use starting in the third quarter of 2026. We recommend preparing for these requirements in advance.
Next Steps:
The European Commission has published a roadmap outlining the timeline and key milestones for the implementation of the various EUDAMED modules. Make sure to review this roadmap to ensure your company is well-prepared. Click here for the roadmap.
Support and Preparation
To prepare for the upcoming launch of the Vigilance module, we recommend the following actions:
- Review and optimize your internal processes for incident reporting.
- Train your staff on the new EUDAMED processes.
- Implement a software solution that simplifies data collection and reporting, ensuring compliance with EUDAMED requirements.
Our team is here to assist you in successfully implementing these measures.
Consequences of Non-Compliance
Manufacturers who fail to meet their obligations risk penalties such as fines or sales restrictions within the EU. It is therefore crucial to meet all requirements on time.
A First Look at the Vigilance Module
We are excited to share with you exclusive screenshots of the Vigilance module from the EUDAMED Playground:













Related Posts