UDI EUDAMED Overview
With the new Medical Device Regulation (MDR 2017/745) and In-Vitro Diagnostic Device Regulation (IVDR 2017/746) of the European Commission, a new requirements for medical devices were introduced. The Unique Device Identification (UDI) aims to globally identify medical devices to enhance traceability, transparency and patient safety.
UDI Impact on Medical Device Manufacturers
In order to place medical devices on the European market, medial device companies are required to comply with these new regulations, including the implementation of UDI. Product-specific UDI have to be maintained and submitted to the European Database for Medical Devices (EUDAMED). Each medical device needs to be assigned to a Basic UDI-DI and needs an unique UDI-DI.
UDI Implementation Timeline
The introduction of the new medical regulations greatly affect the majority of medical device companies and their product portfolio. For this reason, the UDI compliance is successively established and planned to be rolled out by 2027. Thereby, the priority is set according to the risk class of the medical devices.

UDI Project plan
To meet the requirements of the new regulations MDR and IVDR, we have elaborated a step by step roadmap that helps you on your journey to UDI compliance.

UDI Technical Integration
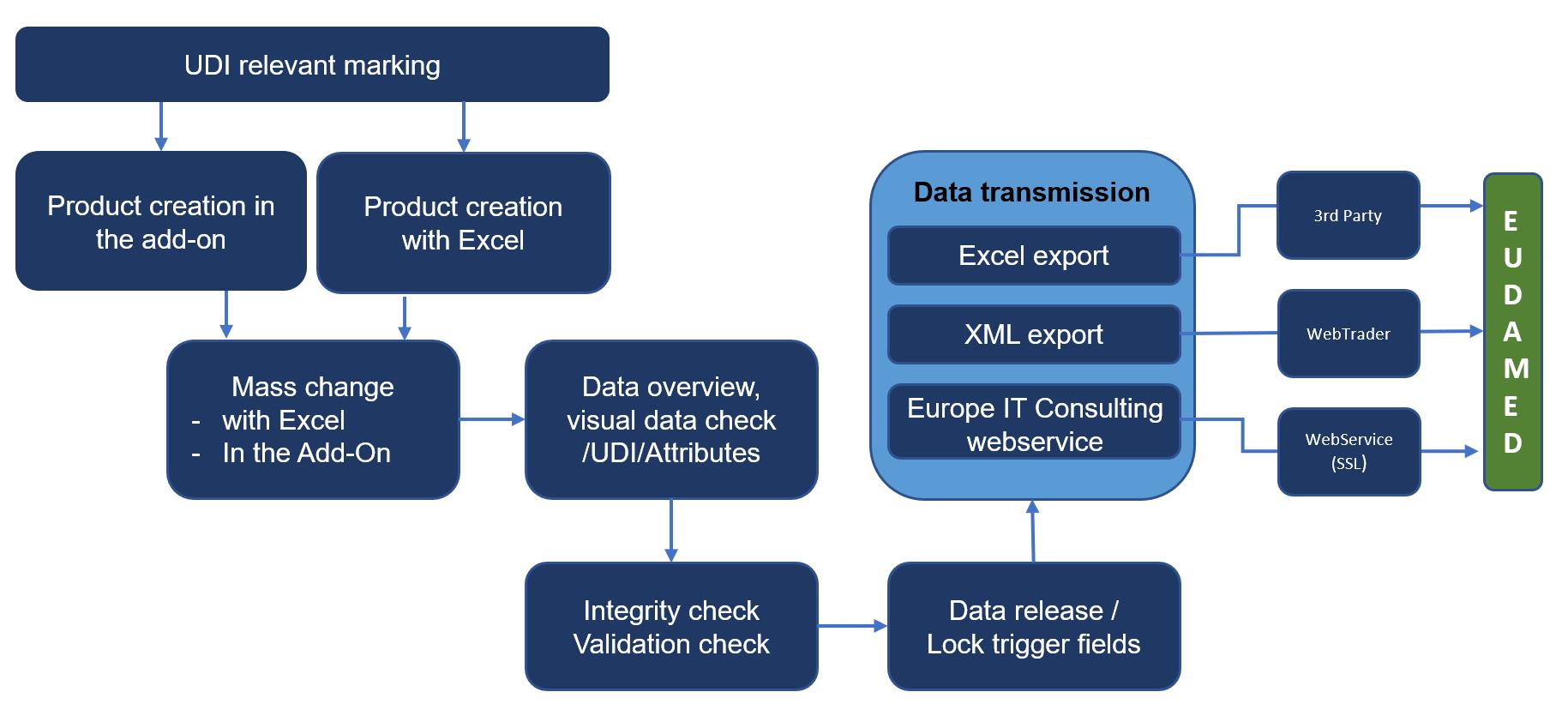
For an effective and efficient UDI data management we have developed an UDI EU SAP Add-On. This solution helps you to maintain, manage and submit your UDI data to the EUDAMED, all in one system. The following illustration represents the UDI data management process in the UDI EU SAP Add-On solution.
UDI Data management
Our solutions for EUDAMED:
If you would like to learn more about UDI implementation, our technical solutions and UDI services, please feel free to contact us at any time.