swissdamed: Medical Device Registration Now Available
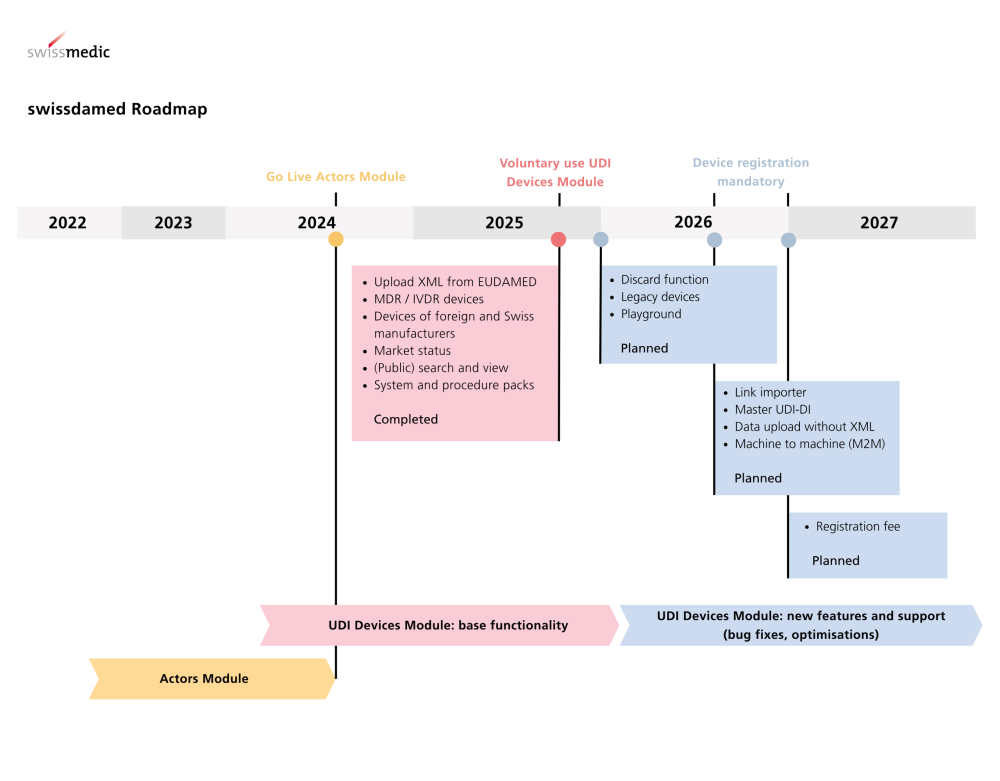
UDI Devices Module Live Swissmedic is expanding the national medical device database with a central functionality. The UDI Devices module in swissdamed has been live since 18 August 2025. From…

UDI Devices Module Live Swissmedic is expanding the national medical device database with a central functionality. The UDI Devices module in swissdamed has been live since 18 August 2025. From…

Europe IT Consulting Successfully Recertified to ISO 9001:2015 On August 6, 2025, Europe IT Consulting GmbH underwent its surveillance audit conducted by QS Zürich AG. The audit report confirms full…
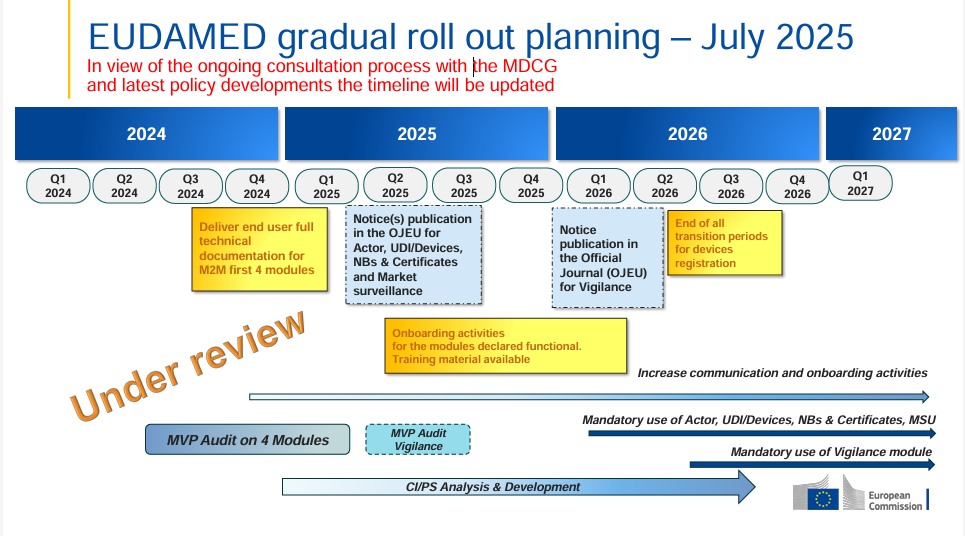
The European Commission has released an updated EUDAMED rollout plan (July 2025) – this time with a clear note: the timeline is now “under review”. This indicates that, as part…
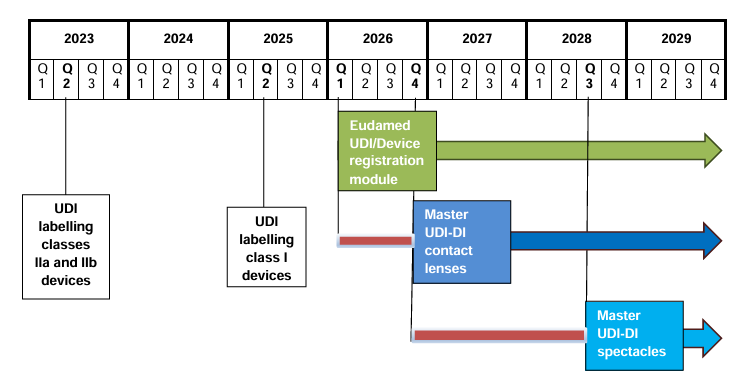
MDCG Publishes Timelines for the Introduction of the Master UDI-DI for Eyewear and Contact Lenses The Medical Device Coordination Group (MDCG) has released its MDCG 2025-7 position paper, setting out…