EUDAMED: Four Modules Mandatory from 28 May 2026
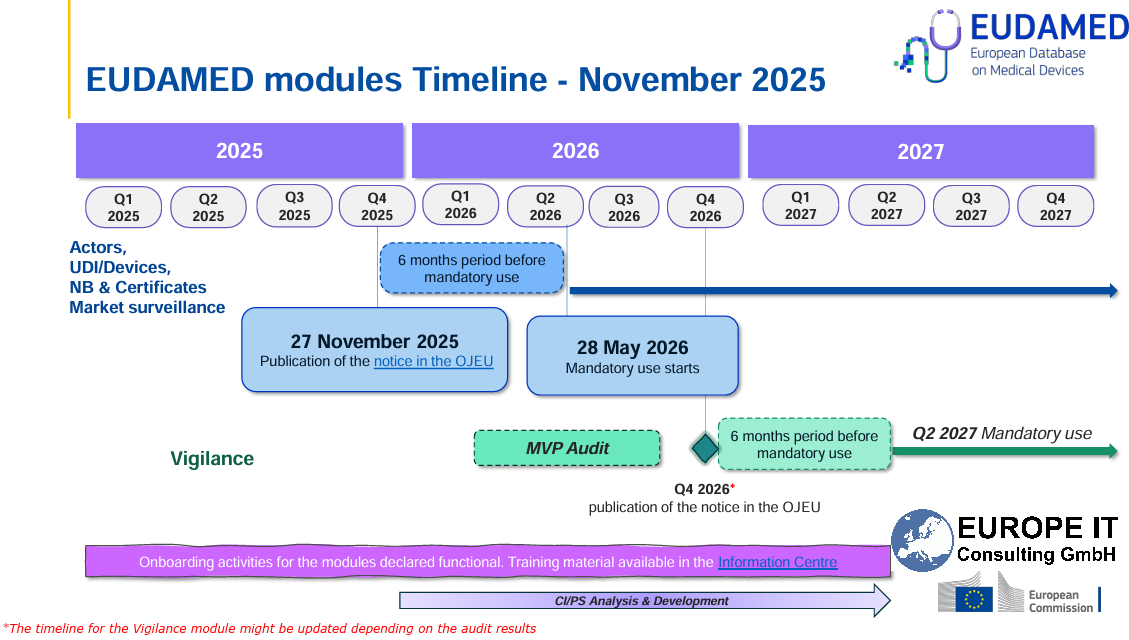
With the publication of a new Commission Implementing Decision in the Official Journal of the European Union on 27 November 2025, the European Commission has officially confirmed that the first four EUDAMED modules are fully functional. This triggers a six-month transition period.
As of 28 May 2026, the use of these modules will be mandatory.
The following modules are affected:
-
Actor Registration
-
UDI / Device Registration
-
Notified Bodies & Certificates
-
Market Surveillance
EUDAMED Roll-out Timeline at a Glance

The current EUDAMED roadmap (as of November 2025) foresees the following milestones:
-
27 November 2025
Publication of the notice confirming the full functionality of the first four modules in the Official Journal of the EU -
28 May 2026
Start of mandatory use of the following modules:-
Actor Registration
-
UDI / Device Registration
-
Notified Bodies & Certificates
-
Market Surveillance
-
-
Q4 2026 (planned)
Separate notice confirming the full functionality of the Vigilance / Post-Market Surveillance module -
Q2 2027 (planned)
Mandatory use of the Vigilance module
The two remaining modules – Post-Market Surveillance & Vigilance and Clinical Investigations & Performance Studies – will only be opened for use when they immediately become mandatory. There will be no extended voluntary phase for these modules.
Registration Deadlines by Device Type

The registration deadlines depend primarily on when a device is first placed on the EU market. Current implementation guidance distinguishes three main scenarios.
1. New MDR/IVDR Devices (“regulation devices”)
Devices that are first placed on the market on or after 28 May 2026:
-
Must be registered in the UDI / Device module before they are placed on the EU market.
-
Without a valid UDI / Device registration in EUDAMED, these products may not be placed on the market.
2. Legacy Devices and Existing MDR/IVDR Devices
Devices that are already on the market before 28 May 2026, either as:
-
Legacy devices under MDD/AIMDD/IVDD, or
-
Devices already compliant with MDR/IVDR,
benefit from a 12-month transition period after publication of the notice in the Official Journal.
-
With the notice published on 27 November 2025, this means:
👉 These devices must be registered in the UDI / Device module by 27 November 2026 at the latest.
3. Devices No Longer Placed on the Market
Legacy or regulation devices that are permanently withdrawn from the market before 28 May 2026, but may still be in the distribution chain:
-
Do not generally need to be registered in the UDI / Device module,
-
unless a vigilance or PMS event occurs that must be reported via EUDAMED after the Vigilance module becomes mandatory (expected from Q2 2027).
What This Means for Manufacturers in Practice
For manufacturers, authorised representatives and importers, the new milestones translate into a concrete need for action in several areas.
1. Secure Actor Registration (SRN)
-
Check whether all relevant economic operators (manufacturers, authorised representatives, importers, and, where applicable, system and procedure pack producers) are already registered and validated as Actors in EUDAMED.
-
Without a valid Actor registration and SRN, device registration and later vigilance reporting will not be possible.
2. Structure UDI and Master Data
-
Prepare UDI and master data so that they can be exported and submitted to EUDAMED in a fully compliant structure – whether via:
-
Excel templates and bulk uploads
-
XML-based submissions
-
M2M interfaces from systems such as SAP or PLM
-
-
Harmonise data sources (ERP/SAP, PLM, existing Excel lists, labelling systems, etc.) to avoid manual rework and inconsistencies.
3. Cluster Your Portfolio by Device Category
-
Clearly distinguish between:
-
New MDR/IVDR devices placed on the market from 28 May 2026 (registration required before placing on the market)
-
Existing MDR/IVDR and legacy devices that will continue to be sold after 28 May 2026 (registration required by 27 November 2026)
-
Devices no longer placed on the market before 28 May 2026 (registration only relevant in connection with vigilance/PMS cases)
-
This portfolio view is essential to prioritise devices, manage workload and ensure that critical products and markets are covered first.
4. Move from “Project” to “Process”
EUDAMED is not a one-off upload exercise. It will become a continuous regulatory process, including:
-
New product launches
-
Changes and recertifications
-
Certificate updates via the Notified Bodies & Certificates module
-
Vigilance and PMS reporting once the Vigilance module is in mandatory use
Manufacturers should define stable, repeatable workflows with clear roles and responsibilities across Regulatory Affairs, Quality, IT and local affiliates.
How Europe IT Consulting Can Support You
Europe IT Consulting supports medical device manufacturers in using the transition period up to May 2026 efficiently – from initial assessment through to automated submissions.
-
EUDAMED Readiness Check
Short, structured assessment of your SRN status, UDI/master data, system landscape (e.g. SAP, Excel, PLM) and overall EUDAMED strategy (Excel-based, SAP add-on, M2M, cloud portal). -
Data and Process Design for UDI & EUDAMED
Design of UDI data models, definition of mandatory and optional fields per authority and set-up of a scalable registration process. -
Technical Implementation
Integration of SAP and other source systems with EUDAMED – for example via our Global UDI framework and add-ons or via a central Global Submission Portal for Excel-to-XML conversion and multi-authority submissions. -
Training & Change Management
Hands-on training for Regulatory Affairs, Quality Management and IT teams on UDI structures, EUDAMED workflows and role concepts.







Related Posts