EUDAMED explained clearly:
Packaging hierarchy vs. Unit of Use DI (and what “Base Quantity” really is)
In EUDAMED, three topics are very often confused: packaging hierarchy (container packages), Unit of Use DI, and “Base Quantity” (in EUDAMED: “quantity of devices”). The reason is usually the same: quantities appear everywhere – but they belong to different logics. This article clearly categorizes the terms and provides a simple checklist for practice.
Why this topic is so often misunderstood
In EUDAMED, two worlds of thinking exist in parallel:
a) Logistics/packaging: carton, outer carton, case – i.e., shipping and storage levels.
b) Use/traceability: what is individually removed/used on the patient – and how is that documented if there is no UDI on the individual item?
If you consistently separate these two logics, it becomes immediately clear: Unit of Use and Base Quantity are not “packaging hierarchy”, but belong to identification and/or use at the unit level.
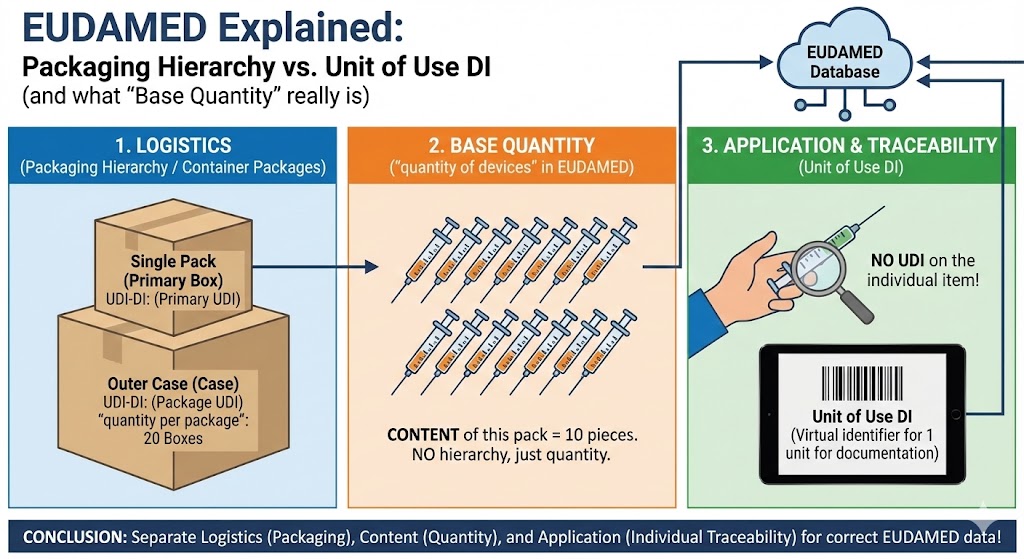
Packaging hierarchy (container packages): “What is packed into which cartons?”
The packaging hierarchy describes the real packaging levels above the sales unit, for example:
Single pack (primary) → carton → outer carton/case
Typically, you maintain per level:
– the Package UDI-DI (which is labeled on this packaging level)– “quantity per package” (how many units of this level are contained in the next higher level, e.g., 20 boxes per outer carton)
Important: this is logistics. This hierarchy only answers the question: “How is the shipping/packaging structure built?”
Base Quantity (colloquially) / in EUDAMED: “quantity of devices”: “How many pieces are IN this pack?”
This field describes the contents of the pack identified by the UDI-DI that you are currently registering.
Example:
– You register the UDI-DI for a box.
– There are 5 caps in this box.
→ “quantity of devices” = 5.
Crucial: this does not build a hierarchy. It is not an additional packaging level. It is only the number of pieces within exactly this packaging level.
Unit of Use DI: “How do you document 1 piece on the patient if there is no UDI on the individual item?”
The Unit of Use DI is a traceability solution for the case that:
– an individual item is used/removed on the patient,
– the individual item itself does not carry a UDI on the item,
– and typically several identical units are contained in a pack.
Then you need an identifier for “1 unit in use”, even though the individual item is not labeled. That is exactly what the Unit of Use DI is intended for.
The most important takeaway for practice:
Unit of Use is not a packaging level. Unit of Use is “single-use/single-removal” identification.
Practical note (as is often the case in day-to-day projects):
In the EUDAMED interface, entering the Unit of Use DI may appear partially optional or can be added later. This means: even if “base quantity”/“quantity of devices” = 5, you may technically be able to save without maintaining Unit of Use. From a business perspective, however, you should always properly assess Unit of Use whenever single removal/single use without individual marking is relevant.
Special case from practice: set/procedure pack with several different “units”
Example: A set consists of different products. The individual products are not sold individually, only the set.
This is typically categorized as follows:
– If different medical devices are marketed together as a set, this set is registered in EUDAMED as a “system” or “procedure pack”.
– The set receives its own Basic UDI-DI and its own UDI-DI at set level.
– The party that combines the components is responsible (system/procedure pack producer).
On the question “multiple Unit of Use DI codes in the set”:
The Unit of Use DI refers to the single use of a unit without individual marking. When you register a set as a system/procedure pack, the set level with its own UDI-DI is the primary focus. The component logic (including potentially different Unit-of-Use references) is then not a topic of the packaging hierarchy, but a set/component topic, which is cleanly covered via the set registration and, if applicable, supplementary internal mapping. Quantity of device is 1 for the set in this case.





Related Posts