
New Schedules for Unique Device Identification (UDI) in Australia
The Australian Therapeutic Goods Administration (TGA) has announced the binding timetables for the implementation of the UDI requirements. The UDI system is part of Australia’s strategy to strengthen patient safety and joins the globally harmonised approach to the precise identification of medical devices.
Background and meaning
The introduction of the Australian UDI system will enable better traceability of medical devices. This supports in particular:
- Post-marketmonitoring: more efficient management of security alerts and recalls
- Patient safety: Improved identification of implants and critical devices
- Regulatory Efficiency: Harmonization with International Standards
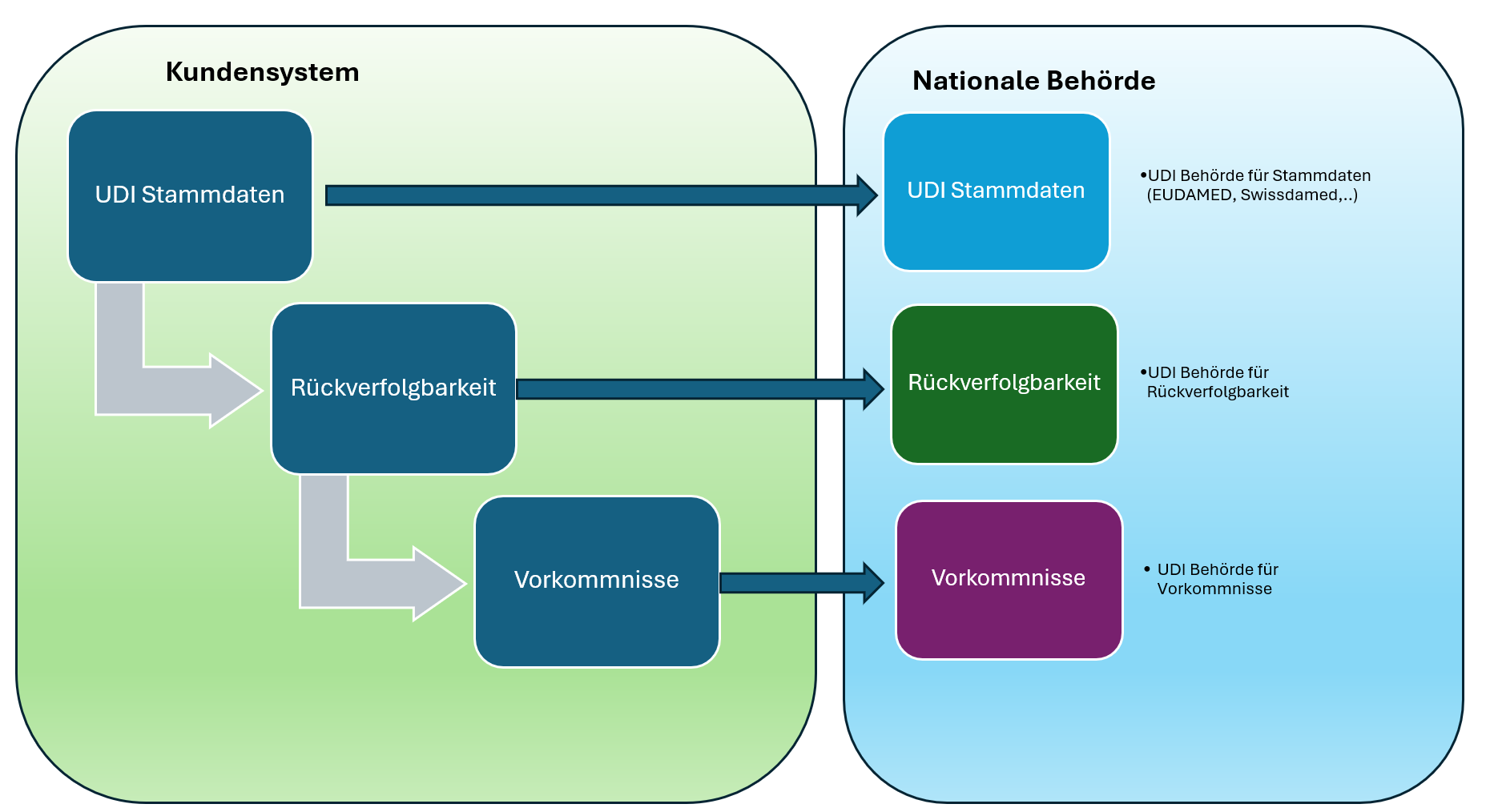
- Data quality: Uniform and reliable device data in the AusUDID (Australian Unique Device Identification Database)
The implementation is risk-oriented over 5 years, starting with high-risk medical devices, in order to grant manufacturers and sponsors sufficient preparation time.
Medical Device Compliance Schedules
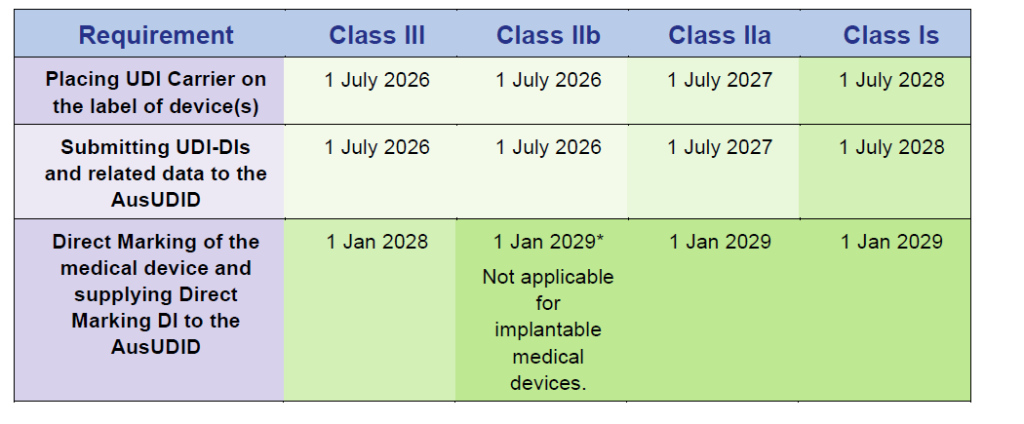
Source: Timeframes for supplying UDI compliant medical devices in Australia, Version 1.0 March 2025
In Vitro Diagnostics (IVD) compliance schedules
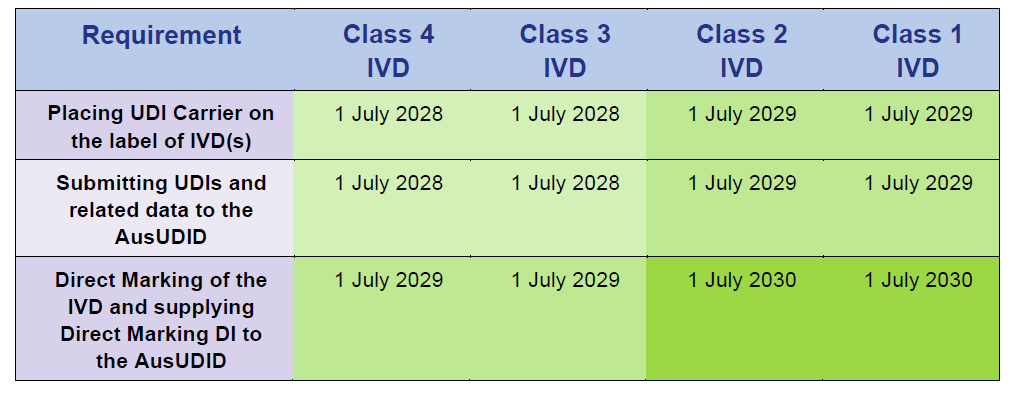
Source: Timeframes for supplying UDI compliant medical devices in Australia, Version 1.0 March 2025
Compliance requirements in detail
The Australian UDI system comprises two main phases of compliance:
Phase 1: Identification and data transmission
- Attaching the UDI carrier to device labels and relevant packaging levels
- Transmission of the UDI-DI (Device Identifier) and associated data to the AusUDID
- Linking the ARTG ID to the UDI records
- Inclusion of the UDI in patient implant cards (for implantable devices)
- Integration of the UDI in TGA reports (adverse events, incidents, market measures)
Phase 2: Direct marking (if applicable)
- Direct marking of reusable devices
- Transmission of the direct marking information to the AusUDID
- Update existing UDI records
Special regulations for existing devices
An important aspect of the Australian UDI scheme concerns devices already manufactured:
For Class III and IIb medical devices only:
- Devices manufactured and labelled before 1 July 2026
- Are still under sponsor control on July 1, 2029
- Must be relabelled UDI compliant by this date
Exceptions:
- Devices already delivered to hospitals, distributors or patients are exempt from UDI requirements for life
- Other device classes (IIa, Is) are excluded from the relabeling requirement
Refurbishment and reprocessing
Particular attention should be paid to remanufactured equipment:
Reprocessing by other manufacturers:
- Applies as new device with all UDI requirements
- Needs new ARTG involvement
- Must meet UDI requirements by the relevant compliance date
Reprocessing by original manufacturer:
- Can be delivered under existing ARTG (with unchanged intended use)
- In the event of a change of purpose: Applies as a new device
- Direct marking requirements apply without exception
Special regulations for EU certificates
Products sold under EU MDD/IVDD certificates in Australia are subject to extended transition periodsaligned with the EU MDR/IVDR transition:
Medical devices under EU MDD certificates:
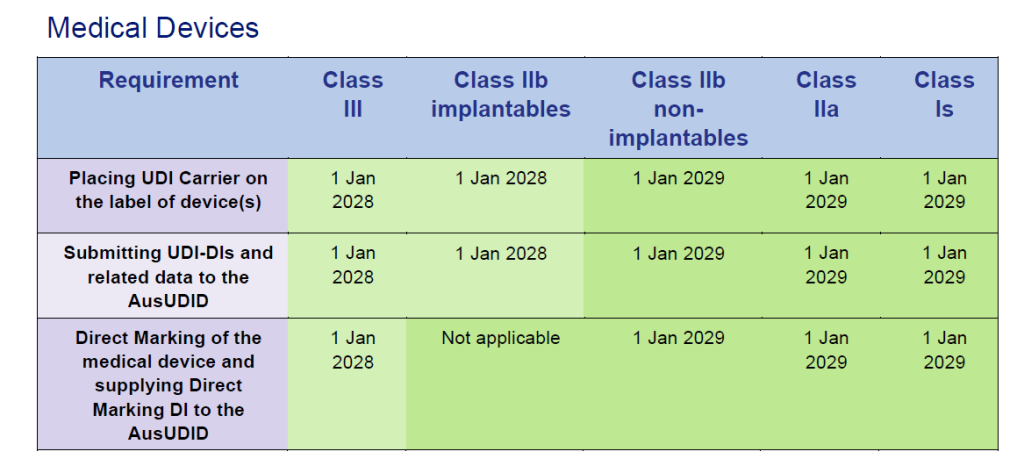
Source: Timeframes for supplying UDI compliant medical devices in Australia, Version 1.0 March 2025
IVD under EU IVDD certificates:
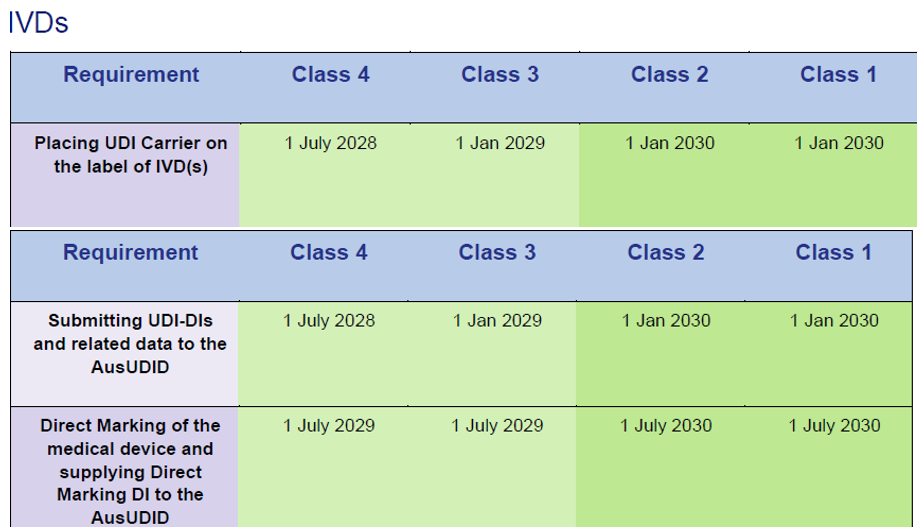
Source: Timeframes for supplying UDI compliant medical devices in Australia, Version 1.0 March 2025
Important: As soon as your products are converted to EU MDR/IVDR certificates, the Australian standard compliance dates apply!
Important notes
- Voluntary implementation is already possible before the binding dates and is recommended
- Existing devices: Class III and IIb devices must be relabeled by July 1, 2029, if still under sponsor control
- Data transfer: UDI data must be transferred within 30 days of being placed on the market
- New applications under MDD: Evidence of EU renewal eligibility required
Our recommendation
Start preparing early:
- Check your device classification
- Prepare UDI Labels
- Test your data transfer methods
- Consider EU Transitional Provisions
Contact us for individual advice on your UDI requirements.
Practical steps to prepare
1. Inventory and classification
- Complete record of all products sold in Australia
- Confirmation of device classification
- Identification of relevant compliance dates
- Examination of existing EU certificates and their period of validity
2. Technical preparation
- Selection of an accredited UDI provider (GS1, HIBCC, ICCBBA)
- Development of the UDI labelling strategy
- Customization of labeling and packaging
- Preparation of direct marking (if necessary)
3. Data management
- Structure of the UDI records with all necessary information
- Testing of data transmission methods (online portal, bulk upload, machine-to-machine)
- Integration into existing ERP and quality management systems
- Training of responsible employees
4. Regulatory Compliance
- Link to ARTG entries
- Preparation of post-market surveillance processes
- Integration with adverse event reporting procedures
- Documentation of all UDI-related processes
Risks of non-compliance
The TGA has announced clear consequences for non-compliance with the UDI requirements:
- Suspension or removal of equipment from the Australian Register of Therapeutic Goods (ARTG)
- Civil penalties under Part 4-11, Division 1 of the Therapeutic Goods Act
- Warnings and fines
Exceptions (Consent to Supply) are only possible in exceptional circumstances and for limited periods of time. Applications must be submitted in good time before the compliance date.
Voluntary early implementation
The TGA expressly recommends the voluntary early implementation of the UDI requirements:
Advantages:
- Reducing confusion among end users (healthcare workers, hospitals, patients)
- Early identification and resolution of implementation issues
- Better distribution of workload
- Competitive advantage through early compliance
Recommendation: If implemented voluntarily, all UDI requirements should be fully met to avoid inconsistencies.






Related Posts