UK: MHRA launches a public consultation

Medical device actors can gives their views and contribute to the future UK´s regulatory framework. Since September 16, 2021, the Medical and Healthcare products Regulatory Agency (MHRA) gives the opportunity…

Medical device actors can gives their views and contribute to the future UK´s regulatory framework. Since September 16, 2021, the Medical and Healthcare products Regulatory Agency (MHRA) gives the opportunity…

Submissions for PECP (Performance Evaluation Consultation Procedure) are accepted since September 3, 2021, by the expert panels in the field of in vitro diagnostic medical devices. What are the expert…
Marked by strong economic development, Asian countries are gradually implementing UDI and regularizing their regulatory environment for medical devices. It is therefore smart to know where these countries are in…

Which details of the critical warnings have to be provided to EUDAMED, to the GUDID ? The Label information, the instructions for use, or both ? What about the contra…
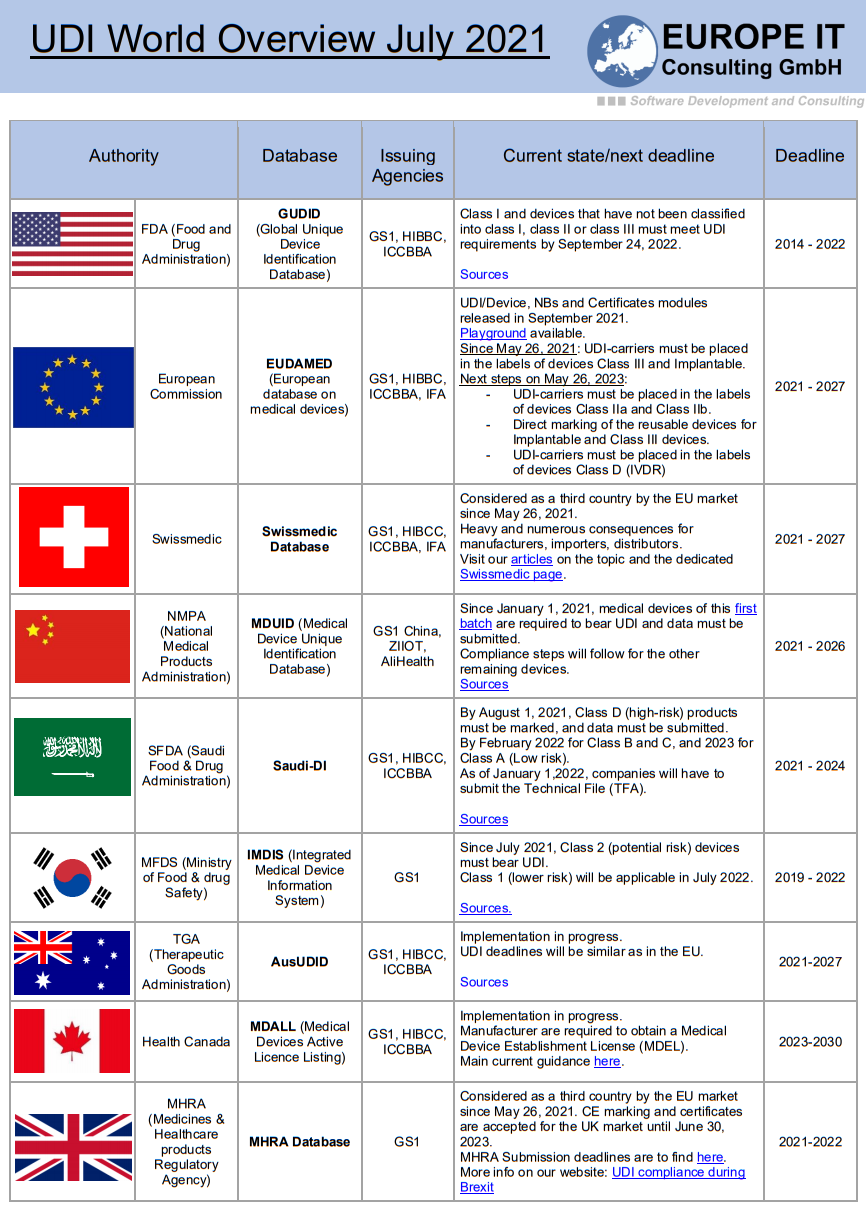
The UDI (Unique Device Identification) field is still evolving and is more or less advanced depending on the country. If you are in any way related to the medical devices…

Our partner Kern AG organizes a free of charges webinar on July 21, 2021 with the participation of Ismail Demiralp as guest lecturer, the CEO of Europe IT Consulting GmbH.Since…

New differing requirements now apply to EU and Swiss medical device manufacturers.As already mentioned in a previous article (MRA between Switzerland and EU no longer valid), Switzerland is considered as…
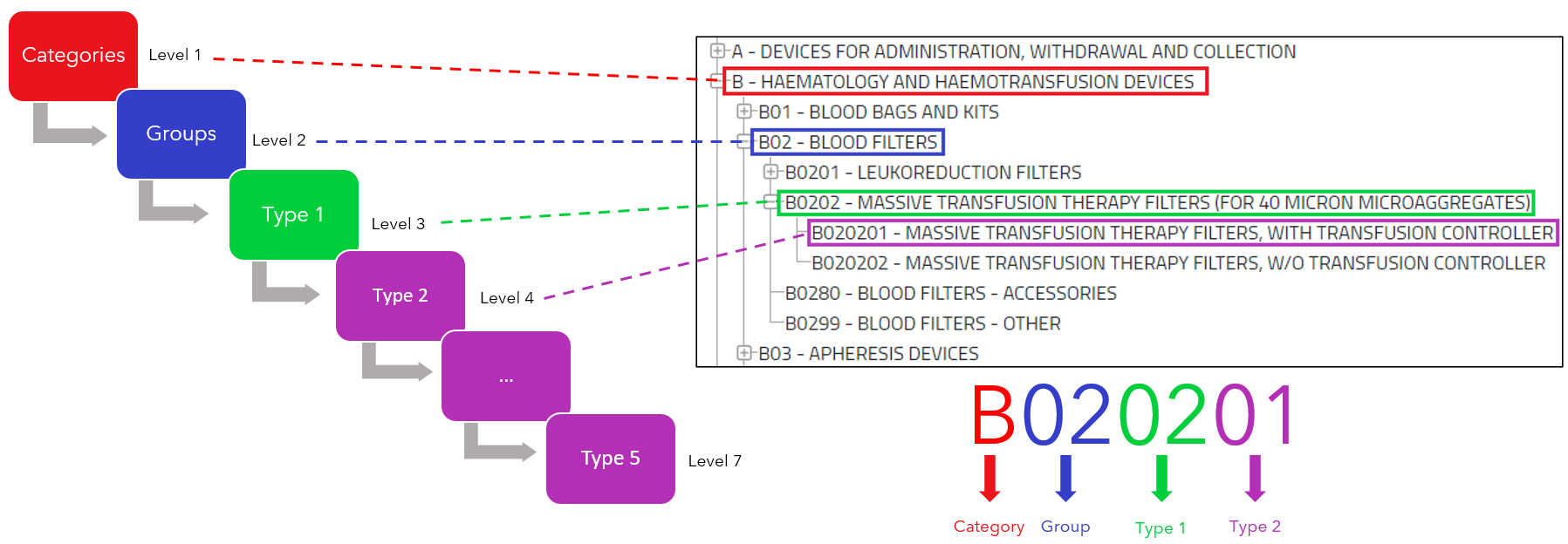
With the implementation of the Medical Device Regulation comes the new EMDN (European Medical Device Nomenclature), as stated in the regulations (Art.26 2017/745 MDR, Art.23 2017/746 IVDR). Review this concept…

With the entry into force of MDR 2017/745 on 26.05.2021, the agreement on mutual recognition of medical devices between Switzerland and the EU lost its validity. Thus, Switzerland is now…
Find out here about the latest status (05/2021) of the UDI implementation for the EUDAMED database.