Definition of a “New Device” under MDR and IVDR

What is a "New Device" according to MDR and IVDR? Introduction The Medical Device Regulation (MDR, EU 2017/745) and the In Vitro Diagnostic Regulation (IVDR, EU 2017/746) have significantly tightened…

What is a "New Device" according to MDR and IVDR? Introduction The Medical Device Regulation (MDR, EU 2017/745) and the In Vitro Diagnostic Regulation (IVDR, EU 2017/746) have significantly tightened…

What is an "Active Device" according to MDR and IVDR? Introduction The Medical Device Regulation (MDR, EU 2017/745) and In Vitro Diagnostic Regulation (IVDR, EU 2017/746) have tightened the requirements…

The live webinar has already ended, but you are very welcome to register to receive the recording and the presentation materials! The EUDAMED database plays a central role in MDR…
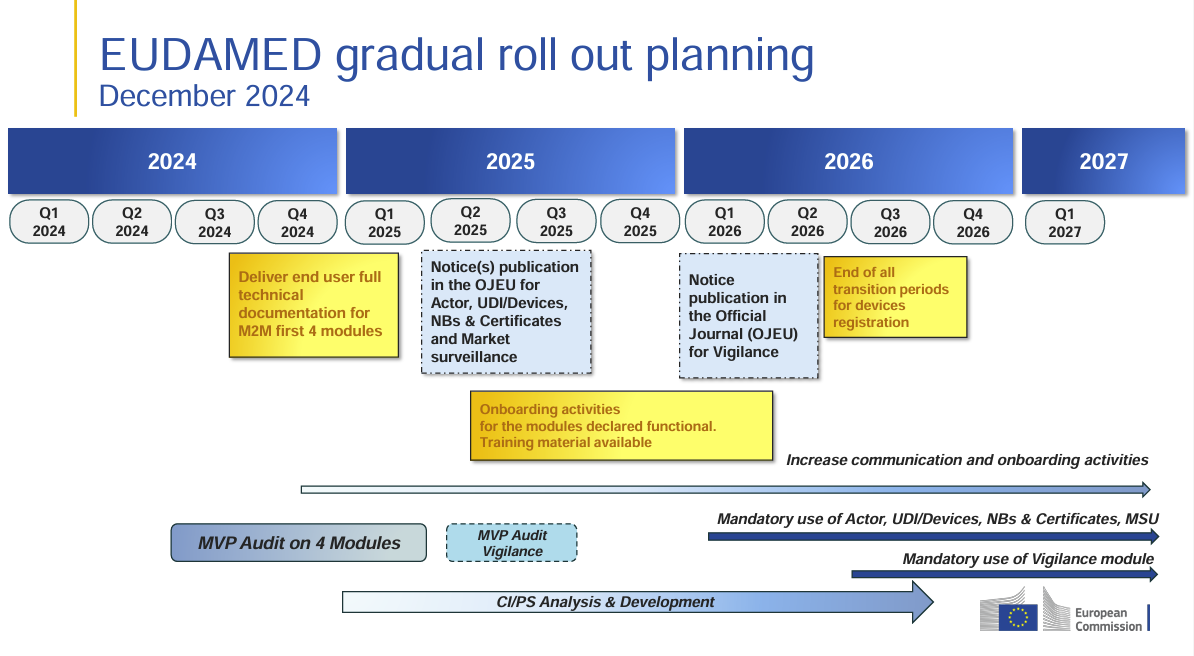
EUDAMED: Updated Rollout Plan for 2025-2027 Released The European Commission has released an updated EUDAMED rollout plan, introducing changes to the implementation timeline and transition periods. Companies in the MedTech…

Revolution or Hype? The MedTech industry faces the challenge of balancing regulatory requirements, efficient production processes, and innovative digital transformation. SAP serves as the backbone of IT infrastructure for many…

Latest Updates from Australia’s TGA The Australian Therapeutic Goods Administration (TGA) has made significant progress in implementing the Unique Device Identification (UDI) system for medical devices. We are pleased to…

FDA Updates GUDID Guidelines: Transition to GMDN Codes Updated FDA Guidelines for the Global Unique Device Identification Database (GUDID) The U.S. Food and Drug Administration (FDA) has released a new…

Dear Customers, Partners, and Friends, During this special time of the year, we would like to extend our warmest Christmas greetings to you. Thank you for your trust and support…

In 2025, numerous exciting events in the field of medical technology and regulatory affairs await us. For companies and professionals in the industry, these trade fairs and congresses provide…

Opportunities, Challenges, and Regulations The United Kingdom (UK) remains a significant market for medical devices despite its exit from the European Union (Brexit). With a growing healthcare sector, investments in…