The introduction of the Medical Device Regulation brought about a significant change: the assignment of Unique Device Identifiers (UDIs) for medical devices.This step has been taken to improve the identification, traceability and monitoring of medical devices while countering counterfeiting. An important milestone on this path is the implementation of the so-called Master UDI-DI.
What is the Master UDI-DI ?
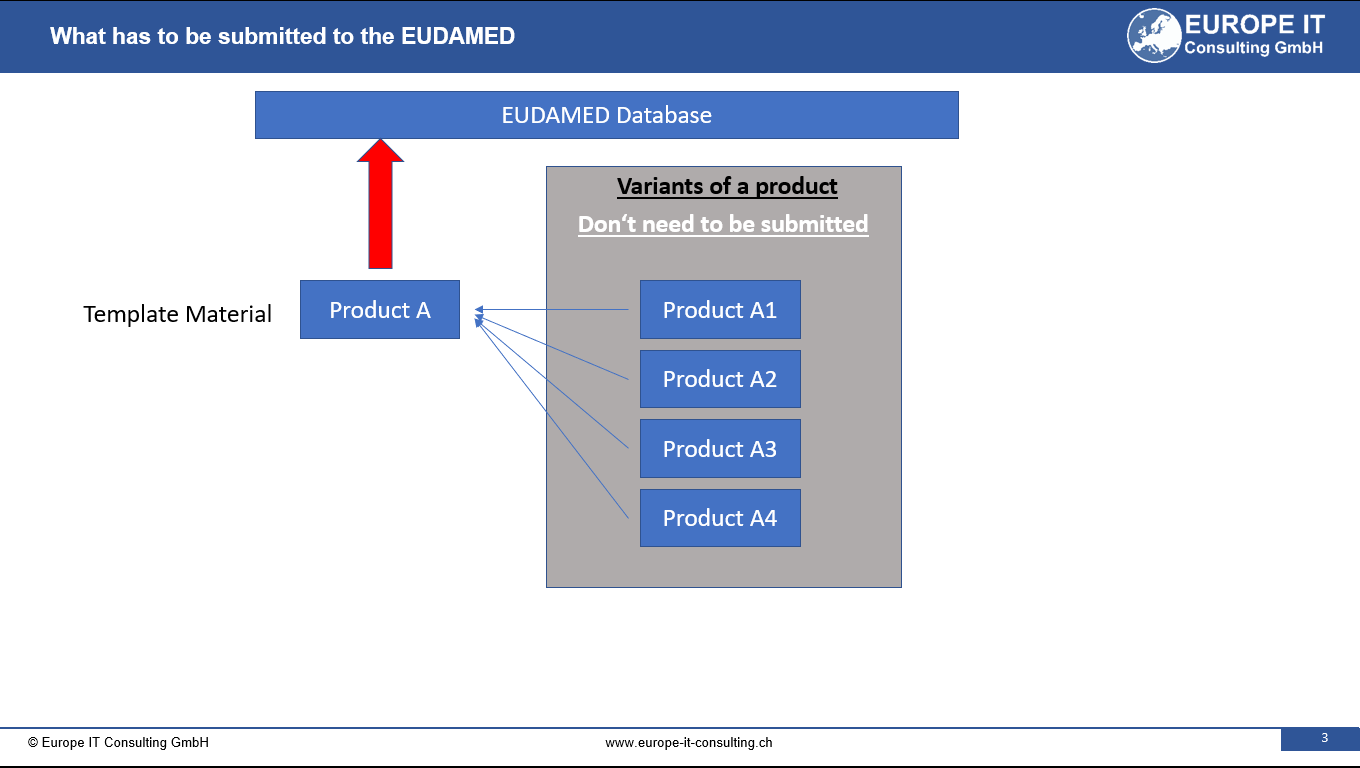
Its main objective is to create an additional level of identification for specific products and medical devices. This innovative approach allows for a more efficient grouping of highly individualized devices, thereby reducing the amount of data entries in the EUDAMED database (UDI module).The main business challenge of the Master UDI-DI lies in the effective categorization of highly individualized devices. This approach not only results in a reduced amount of data entry in the EUDAMED database, but also supports the provision of more detailed product information using additional application identifiers for clinical specifications.
Implementation and benefits
The master UDI-DI is applied as a product identifier (PI) in the UDI barcode on the packaging or label of the product. The precise specifications and requirements for the Master UDI-DI may not have been finalized by EUDAMED (European Database for Medical Devices) at this time.
The first product categories that require the Master UDI-DI include contact lenses, spectacle lenses and ready-to-use reading devices. Nevertheless, it is important to emphasize that the list of products with a high degree of individualization could be expanded in the future.
 The implementation of the Master UDI-DI may seem like an additional hurdle, but it plays an essential role in the process of identification and traceability of medical devices.
The implementation of the Master UDI-DI may seem like an additional hurdle, but it plays an essential role in the process of identification and traceability of medical devices.
This measure underlines the ongoing pursuit of higher safety, quality and efficiency in the field of medical devices.
Current developments in the Master UDI-DI in EUDAMED
With the latest update of the EUDAMED test environment (Playground), an important step has been taken towards the concrete implementation of the master UDI-DI functionality. Manufacturers can now register certain highly individualized products – such as standard contact lenses, dimensionally stable RGP lenses or custom-made spectacle lenses – under a Master UDI-DI .
This extension allows companies that sell large quantities of these product types to use only a single master UDI-DI for an entire product family – instead of entering each individual variant separately.

New menu item for Master UDI-DI in the EUDAMED test environment.
When is a Master UDI-DI required?
The obligation to create a master UDI-DI is triggered by certain criteria:
-
The products belong to the same generic product group.
-
They have individual specifications (e.g., visual acuity, curvature, cylinder).
-
They are classified according to the same MDR set of rules.
-
They are subject to the same risks and clinical assessments.
For example
, a contact lens manufacturer may register all variants of a particular type (e.g., monthly lenses of different prescriptions) under a master UDI-DI.

Selection of product types – e. B. “Standard soft contact lenses”

selection with note: “Registration is not possible currently …”

Focus on lens types, incl. “Standard soft contact lenses”
💡 Note: This option is currently only available in the test environment. The function is expected to go live after further tests have been completed.
EUDAMED Input Mask: Same but Expanded
Although the menu item changes when selecting a master UDI-DI, the underlying fields and requirements correspond to those of other MDR products. The input mask thus remains familiar, which makes it easier to familiarise yourself with it.
Enter the master UDI-DI and associated output point.
Features such as single-use, reuse and sterilization before use.


Our support for manufacturers
Europe IT Consulting actively supports manufacturers in the correct implementation of the Master UDI-DI obligations:
-
Checking the suitability of products for a Master UDI-DI
-
Provide compliant Excel templates for UDI capture
-
Automated XML generation for EUDAMED
-
Test transmissions in the EUDAMED playground environment
-
Training and individual advice






Related Posts