Europe IT Consulting introduces the Global Submission Portal with a focus on FDA eMDR
New cloud platform combines eMDR, GUDID and EUDAMED in a single system
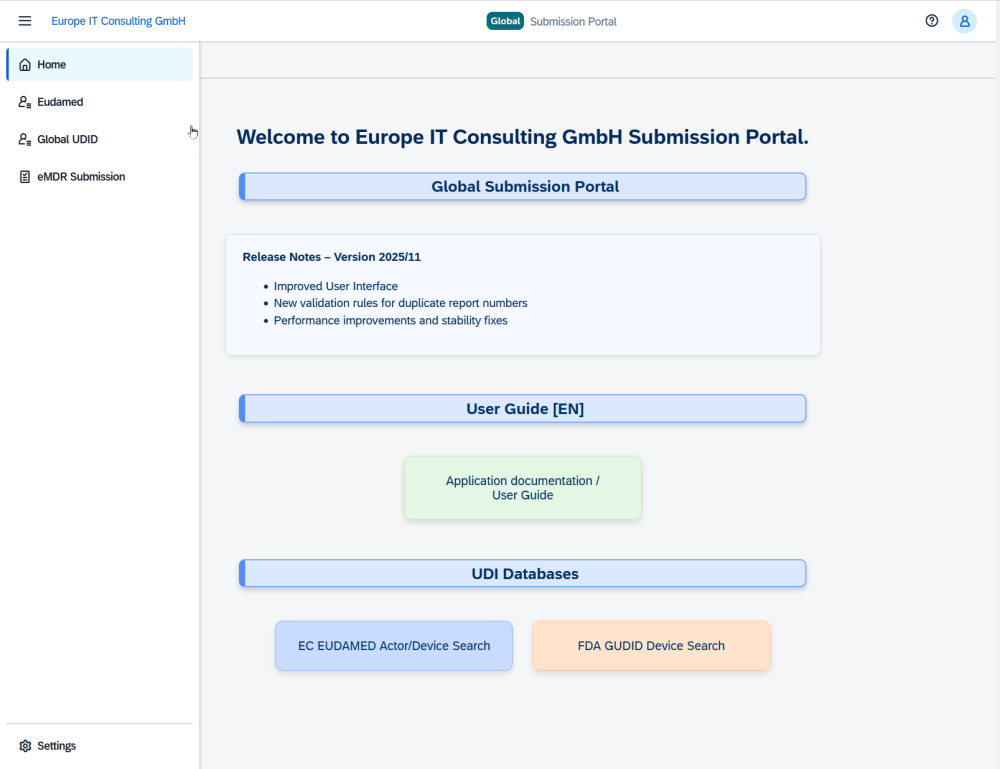
With the Global Submission Portal, Europe IT Consulting is launching a cloud platform that significantly simplifies the complex process of FDA eMDR reporting. In addition, the solution supports UDI for FDA GUDID and EUDAMED, making it a central platform for global submissions to selected authorities.
Medical device manufacturers face the challenge of managing reports and UDI data in different formats and portals. Especially in the vigilance area, time pressure is high – and errors can have a direct impact on deadlines and compliance.
Why FDA eMDR is in focus
Electronic reporting of incidents to the FDA (Electronic Medical Device Reporting, eMDR) is technically demanding: HL7 ICSR structure, ESG Submission Gateway, multiple ACK messages and strict validations. Many companies are still working with manual uploads and separate tools in this area.
Europe IT Consulting directly addresses this pain point: the Global Submission Portal is designed so that eMDR cases can be processed in a structured, automated and fully traceable way – without the specialist departments needing HL7 or XML know-how.
FDA eMDR as a core module in the Global Submission Portal
The FDA eMDR module is one of the three central building blocks of the Global Submission Portal:
-
Excel-based case capture
Specialist departments enter all relevant information (e.g. Adverse Event, Product Problem) in a structured Excel template aligned with FDA requirements. -
Automatic conversion & transmission
Based on the Excel data, a valid HL7 ICSR XML file is generated in the background and transmitted to the FDA via an M2M connection through the ESG Submission Gateway – without any manual handling in the eSubmitter tool. -
ACK handling & status overview in the portal
The acknowledgements (ACK messages) returned by the FDA are processed automatically and displayed in the portal with clear statuses – from “Uploaded” and “Processing” through to “Success” or “Failed”. -
Validation errors & download center
If validation errors occur, they are displayed with details (e.g. section, XPath, authority message). Result files and authority responses – such as FDA ACKs – can be downloaded either collectively as a ZIP file or individually.
This makes the Global Submission Portal the central interface through which vigilance and regulatory teams can manage their eMDR reports from initial data entry through to final confirmation.
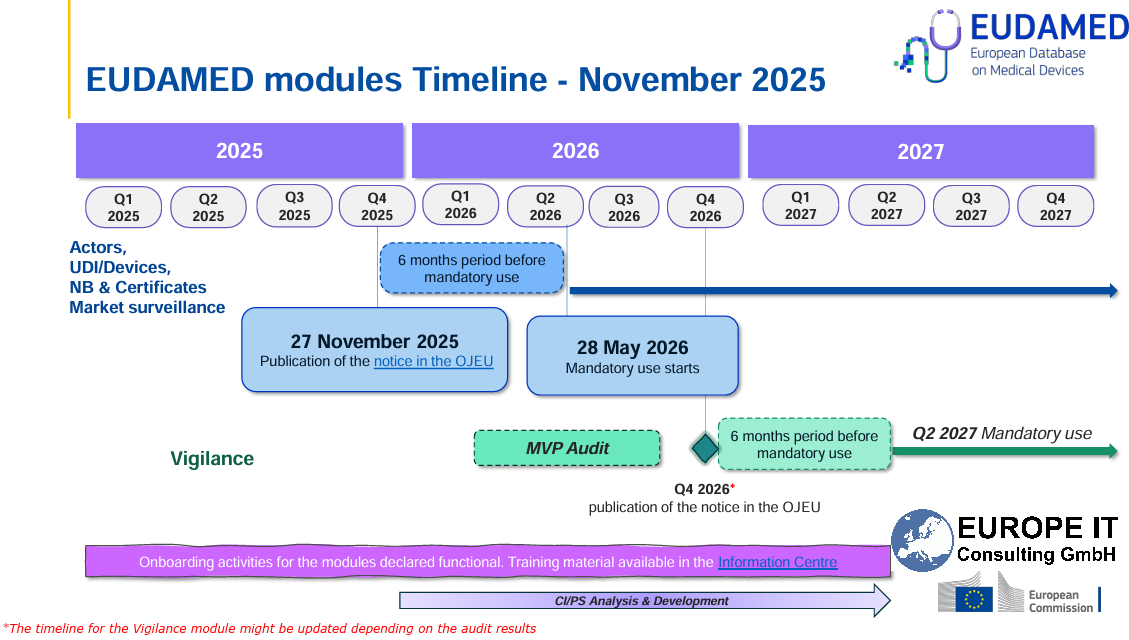
Additional modules: UDI submission to FDA GUDID and EUDAMED
In addition to eMDR, the Global Submission Portal covers two further key compliance areas:
-
FDA GUDID – Support for submitting UDI data to the Global UDI Database for the US market
-
EUDAMED – EU MDR-compliant UDI submissions to the European Database on Medical Devices
The same principles apply here as well: Excel-based upload, automatic validation and transparent status tracking of all submissions.
Learn more & request a demo
The Global Submission Portal is available now.
For more information, please visit:
Product page: https://www.europe-it-consulting.ch/global-submission-portal/?lang=en








Related Posts