Global UDI data management for SAP – secure, fast & globally compliant
Would 💬 you like to transfer UDI data automatically from SAP? 👉 Request a free demo now and experience the solution live. Request Demo
As a manufacturer of medical devices, you face the challenge of transmitting UDI data correctly, completely and on time to international authorities such as EUDAMED (EU), FDA GUDID (USA ) , SWISSDAMED (Switzerland) or SFDA (Saudi Arabia).Our Global UDI SAP add-on solution simplifies just that: you maintain your product data centrally in SAP – and transfer it automatically to all relevant UDI databases.
✅ No more Excel exports
✅ Automatic validation and transfer of your UDI data
✅ Legal compliance worldwide – without additional IT effort
Flexible UDI data management directly in SAP – on-premise & cloud
Our solution is fully integrated with SAP – whether S/4HANA, SAP NetWeaver 7.50+ or the Private SAP S/4HANA Cloud. You work where your data already is – without system breaks or external tools.
🔹 Modular structure: Activate only the government plugins you actually need
🔹 Excel upload included: Import UDI data conveniently via our structured UDI Excel upload template
🔹 SAP Fiori / SAP UI5 interface: Intuitive operation – even for non-SAP users in the web browser labeling interface:
🔹 Compatible with OPAL labelmanagement for direct use of your UDI data on labels
Your advantages at a glance – Global UDI SAP Add-On
 Global UDI SAP Add-On |
|
|
| ✅ SAP compatibilitySeamless integration into S/4HANA (OnPremise/Cloud) and SAP NetWeaver 7.50+ – without external systems&# |
||
| ✅ Easy operation for all rolesIntuitiveinterface based on SAP Fiori & UI5 – even for non-SAP users |
||
|
|
||
|
|
||
|
|
||
|
|
||
Would you also like to use these advantages for your company?
👉 Get a 30-minute live demo of how easy UDI compliance can be.
🎥 UDI compliance explained simply – in 2 minutes
In our video you can see how easy and efficient UDI data management can be in SAP.
Experience the most important functions and advantages of the solution – explained in a compact and understandable way.
✅ Ready to use – without complex integration
✅ Modular and individually adaptable (authorities, processes, budget)
✅ Compatible with S/4HANA, SAP NetWeaver and Private Cloud
Watch the video 👉 now and understand in a few minutes how you too can automate your UDI processes.
Supported by S/4 HANA, SAP Netweaver 7.50 and higher, as well as the Private S/4 HANA Cloud, our solution offers a frontend base on SAP Fiori and SAP Ui5 technology, accessible via the web browser.
Why a strong UDI solution is crucial – and how it benefits you
As a manufacturer of medical devices, you are responsible for product safety, compliance with authorities and efficient processes.
Our Global UDI solution helps you to combine all this in one system :
🔹 Secure and auditable: All UDI data is traceable, validated and documented by the authorities at all times
🔹 Future-proof: Support global requirements – EUDAMED, FDA, SFDA, TGA, NMPA etc
🔹 Efficiency gains: Less manual processes, shorter time-to-market, lower error rate
🔹 Central data maintenance – global effect: You control your data, not Excel
With our SAP add-on, you not only gain compliance, but also control, transparency and speed.
Clear structure & access control – with business units in SAP
In complex companies with multiple locations or product areas, our business unit function ensures order:
🔹 Separation by business area: Assign UDI data specifically to individual business units – e.g. B. by location, product type or responsibility
🔹 Tailored access rights: Each user sees only the data that is relevant to their role SAP-compliant
🔹 Built on the SAP standard authorization model – without additional system changes
🔹 Central maintenance, decentralized responsibility: Data can be managed globally and shared locally
In this way, you retain control, avoid errors and ensure data protection-compliant processes.
For efficient organization and easy access control, you can assign all your materials to different business units, making it easier to view and maintain specific product groups.
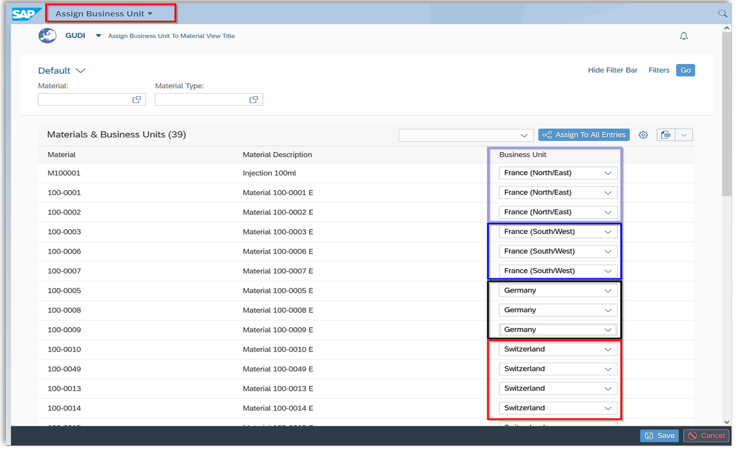
Business Units in our Global SAP Add-On
UDI release with system – flexible, secure, traceable
Our intelligent UDI approval process can be adapted exactly to your internal processes – whether you are working with two or ten approval steps.
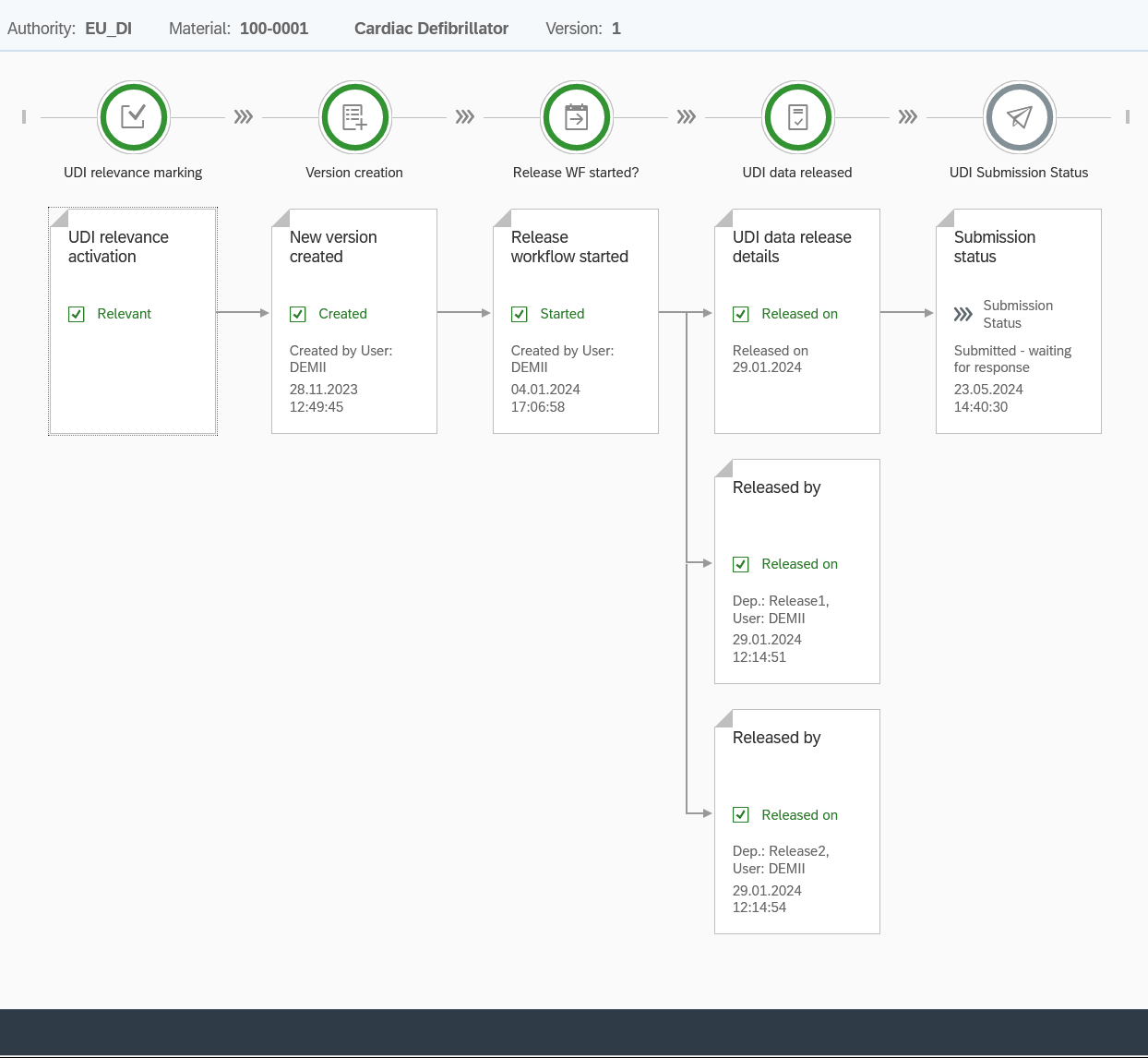
UDI Release Workflow
Features:
🔹 Full control: Set who shares – in what order and with what permissions
🔹 real-time notifications: Via email or directly in the SAP Fiori Launchpad – you won’t miss a task
🔹 Visual Dashboard: See at a glance all open, running and completed shares
🔹 Automatic reminders: No delays – all participants are reminded in a timely manner
🔹 Audit trail included: Complete traceability of every change – audit-proof and compliant
🔹Seamless integration: Works in conjunction with your existing SAP workflows and authorization concepts. This ensures that your UDI data is released correctly, completely and in a timely manner – and keeps track of it at all times.
Maximum flexibility: government plugins as needed
With individual plugins for each UDI authority, you can keep track and avoid unnecessary complexity.

🔹 Just what you need:
only the plugins that are relevant to your target markets (e.g. EUDAMED, FDA, SFDA, NMPA, TGA)
🔹 User-centered view:
Each user only sees the countries and tasks relevant to them
🔹 Minimised maintenance effort:
Existing product data is automatically transferred to country-specific formats
🔹 Intuitive user guidance:
Each authority has its own, clearly structured Fiori group in the dashboard
This keeps your solution lean, efficient and scalable – even with growing regulatory requirements worldwide.
Every change is traceable – with an integrated audit trail
In medical technology, it is not only what you do that counts – but how well you can prove it.That is
why our UDI solution is fully auditable and meets all requirements for traceability and compliance.
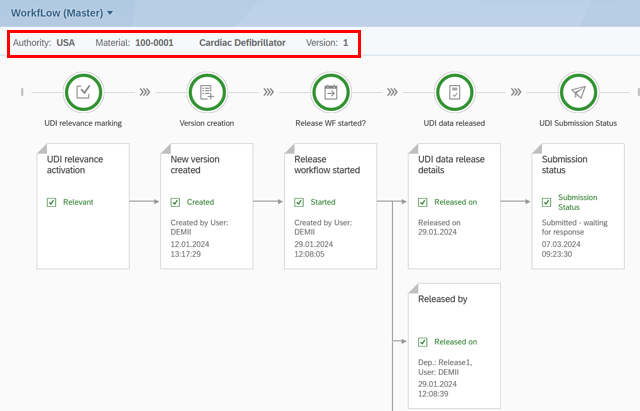
UDI Release Workflow
🔹 Complete logging:
All changes to UDI data are automatically recorded – with timestamp and user assignment Workflow
🔹 History:
Track step-by-step how and by whom a record was processed
🔹 Audit-ready:
Ideal for external inspections and internal QA audits – no additional follow-up required
🔹 Integrated instead of retrofitted:
All audit features are included by default – no extra hassle
In this way, you can document your processes in an audit-proof manner – and at the same time gain clarity and control in day-to-day business.
Easily migrate UDI data with Excel & XML templates
Whether you want to restart or transfer existing data into the system:
our solution supports you in a structured, secure and efficient way – even without SAP know-how.
🔹 Excel templates for a quick start: Fill your UDI data conveniently into a structured template – the upload is automatic and validates
🔹 XML for standardized government interfaces: Structured UDI data can be processed directly or transferred automatically
🔹 Compatible with non-SAP systems: Our templates also work for companies without SAP – ideal for service providers or external partners
🔹 Minimum training, maximum control: No training required – your data is ready for immediate use
Whether migration, initial filling or data correction – our tools make the transition smoothly and without compromising on data quality.
.

UDI Upload Template
Full data control – through flexible UDI data export
Your UDI data does not remain “trapped” in the system, but can be exported at any time – structured export in Excel or XML format including:
🔹 Excel export for maximum flexibility
-
Easy to read, check and process
-
Ideal for internal approvals, audits or data analysis
-
Compatible with Microsoft Office, SAP and third-party tools
🔹 XML export for structured government data
-
Machine readable & standardized for automatic transmission
-
Compliant with regulatory requirements (e.g. FDA GUDID, EUDAMED)
-
Seamlessly integrates with existing IT environments
This allows you to maintain full transparency, post-processing options and automation potential at all times – whether for authorities or internal processes.
Easily import UDI data – in 3 clear steps
With our validated Excel template, you can quickly, securely and user-friendly transfer your existing UDI data to the system.Ideal
for initial filling, mass changes or system migrations:
① PreparationEnter your UDIdata into the structured Excel template – no programming required.
②. Validation
The software automatically checks the file for completeness, format errors and regulatory requirements
③ Upload
One click is enough: The data is transferred directly to the UDI module and is ready for immediate use.
🔁 Can also be used for subsequent updates or corrections – simple, secure, transparent.
UDI labels at the touch of a button – with OPAL labelmanagement
Your UDI data is not only relevant for authorities – but also for labels, packaging and delivery processes.
Our solution is fully compatible with the OPAL labelmanagement system.
🔹 Direct access to UDI data from SAP: No export, no double entries
Automatically generate labels: Barcode, GTIN, production data etc. can be directly integrated
Optimize printing processes: Uniform templates, automated processes,fewer errors
🔹 100% UDI-compliant: Your labels meet all regulatory requirements
In this way, you can combine regulatory compliance with efficient labelling in production and logistics – without any media disruption.
Learn more about:
MDR white paper – everything you need to know about UDI compliant labelling
Structured towards the goal – project implementation with plan & tools
Our Global UDI solution is not only technically mature, but also prepared for a smooth implementation in your company. With well thought-out templates, validated documents and a clear project approach, you can bring your UDI processes to life efficiently and securely.
🔹 Default Project Templates
Our pre-configured templates provide you with a clear step-by-step structure for the entire UDI implementation – from planning to implementation to training. This avoids project delays and ensures that all regulatory requirements are taken into account.
🔹 Prefabricated CSV and validation documents (GAMP5 compliant)
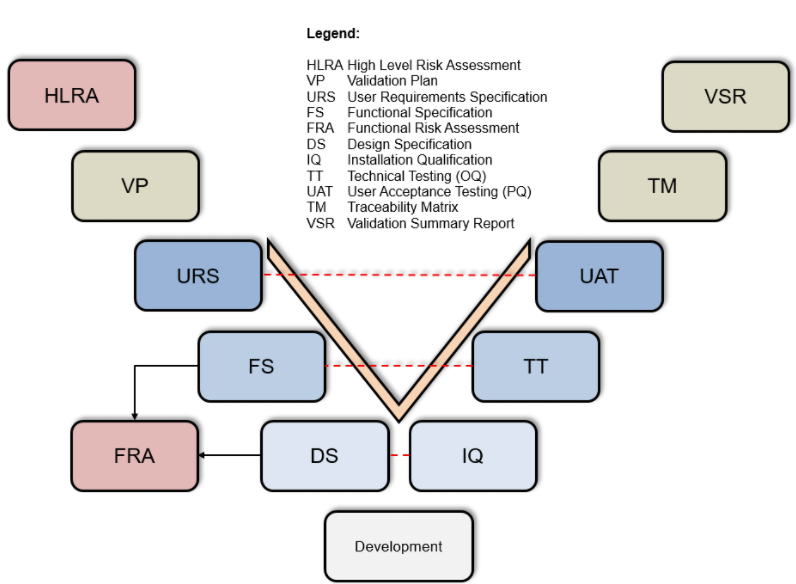
V-Model CSV Validation
Use verified templates for the structured collection of your UDI data – including validation steps according to
the V-model.This ensures data quality, traceability and compliance from day one.
🔹 Minimized effort – maximum impact
The combination of project structure, tools and experience reduces internal effort and accelerates implementation – without any loss of quality.
✅ This ensures that your UDI project remains plannable, traceable and auditable – and you can concentrate on your core business.
Seamless UDI data transmission – compliant with authorities worldwide
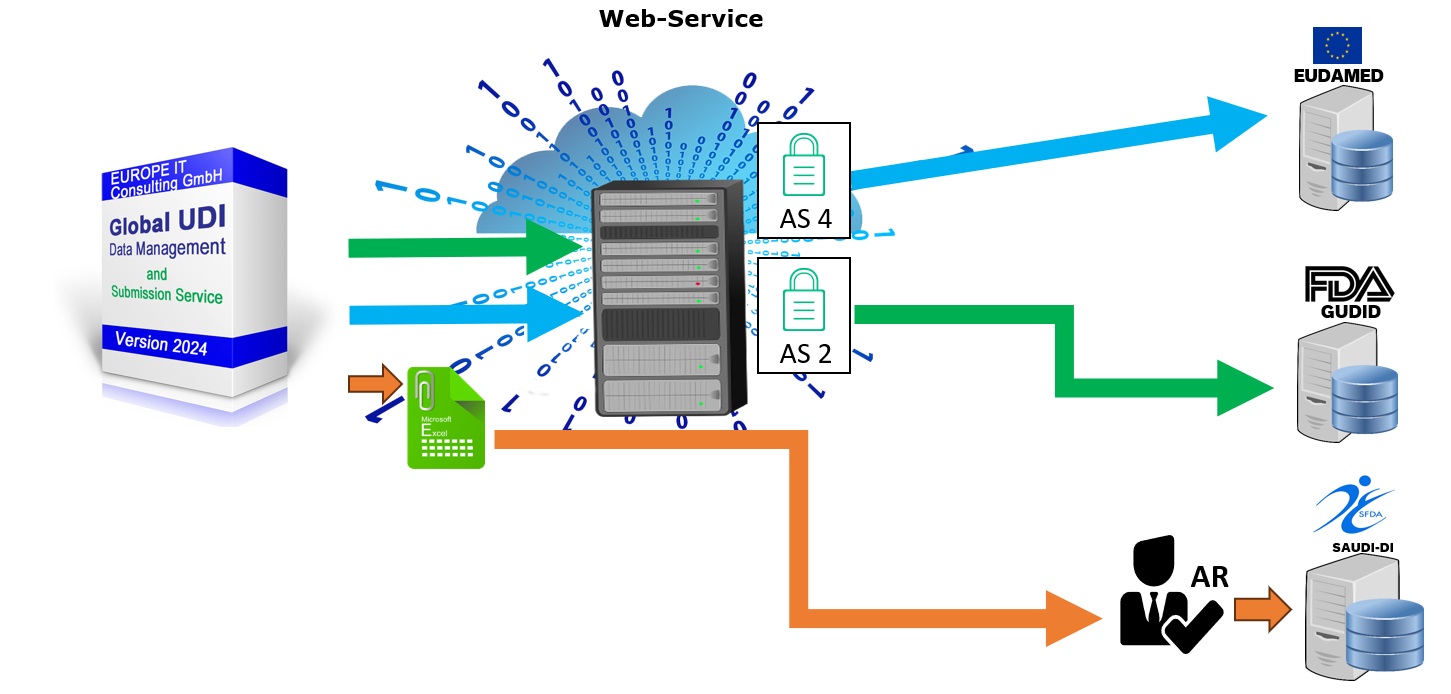
UDI AS2/AS4 Data submission
With our Global UDI solution, you transfer your UDI data fully, validated and automated to all relevant regulatory authorities – including EUDAMED, FDA GUDID, SFDA, TGA, NMPA and more.
🔹 Technically integrated & regulatory compliant
-
Support for all common protocols: AS2, AS4
-
Data formats such as HL7 XML for structured, authority-compliant communication
-
Seamless connection to your SAP systems and existing processes
🔹 Validated before shipment
-
Built-in test modules ensure that your UDI data is complete, correct and compliant
-
Errors, inconsistencies or missing mandatory fields are detected in advance – before they lead to problems
🔹 Automated workflows with full traceability
-
Every step – from creation to validation and approval to submission – is digitally controlled and logged
-
This enables you to meet the highest requirements for data integrity, audit capability and compliance
✅ Your advantages: No manual upload, no returns, no risk – but a secure, lean and regulatory-protected transfer process.
🏆 Satisfied customers from the MedTech industry – in use worldwide
Our Global UDI solution is field-proven and has been successfully used for years by leading medical technology companies – both for FDA GUDID and for EUDAMED.
Since 2016, numerous manufacturers have been using our SAP add-on to manage, validate and transmit their UDI data directly in SAP to the respective authorities – automated, auditable and independent of third-party systems…
 |
|
|
|
Interested?
No SAP solution? No problem – with our UDI Excel templates
You want to manage UDI data and transfer it to EUDAMED, FDA or other authorities – but do not have an SAP system in use?
Our UDI Excel templates do just that: structured, secure and compliant.
🔹 UDI capture without SAP:
All the necessary information can be entered directly into our multi-part Excel template – clearly structured with tabs for the different data areas.
🔹 Always up-to-date & regulatory safe:
Our templates are regularly updatedto meet the latest requirements of the authorities – e.g. new mandatory fields, format specifications or upload specifications.
🔹 Prepared for authorities:
The data from the Excel templates can easily be reused for uploading to UDI databasessuch as EUDAMED, FDA GUDID or SFDA .
🔹 Ideal for service providers, suppliers and non-SAP users:
even partner companies or smaller organisations can prepare UDI data independently – and remain compliant with you.
✅ Conclusion: With our Excel templates, you get a cost-effective, flexible and ready-to-use solution for UDI data management – without SAP installation.
UDI news and specialist articles
Current status of global UDI systems
The field of UDI (Unique Device Identification) is still evolving and is more or less advanced depending on the country.|||UNTRANSLATED_CONTENT_START|||Wenn Sie in irgendeiner Weise mit dem Geschäft mit medizinischen Geräten zu tun haben, kann es für Sie relevant sein, über die Vorschriften und Fristen auf der ganzen Welt auf dem Laufenden zu bleiben.
|||UNTRANSLATED_CONTENT_END|||
Webinars on the theme of Unique Device Identification
Here you will find a list of our upcoming online events, as well as a selection of past webinars whose recordings and related materials are available to you.Our webinars are specifically designed to give you in-depth knowledge and practical insights into the world of UDI. Whether you are new to this field or have already gained experience and would like to deepen your knowledge, our webinars offer something for everyone.
https://www.europe-it-consulting.ch/index.php/udi-webinaries/
Your contact persons
Our dedicated team consists of highly qualified consultants and expertswith extensive experience in the UDI industry. Passionate about technology and innovation, we strive to always provide our customers with the best solutions and first-class service.


Here you can request your quotation?
