Swissdamed UDI mandate begins July 2026

The Swissdamed platform continues to take shape – and with it the regulatory obligations for manufacturers and distributors of medical devices in Switzerland and Liechtenstein. A central module is now…

The Swissdamed platform continues to take shape – and with it the regulatory obligations for manufacturers and distributors of medical devices in Switzerland and Liechtenstein. A central module is now…

We are thrilled by the enormous interest in our free expert webinar on the EUDAMED database! With over 700 registrations, we clearly felt how relevant and important this topic is…

New EUDAMED FAQ: Comprehensive Answers to Your Questions About the EU Medical Device Database We are pleased to announce that we have expanded our website with a new, comprehensive FAQ…

The importance of the EUDAMED packaging system for the regulation of medical devices in the EU The EUDAMED packaging system is an indispensable part of the broader EUDAMED system, which…

The EU Medical Device Regulation (MDR) introduced stricter rules and clearer responsibilities for all actors involved in the life cycle of medical devices. One area that raises particular questions is…
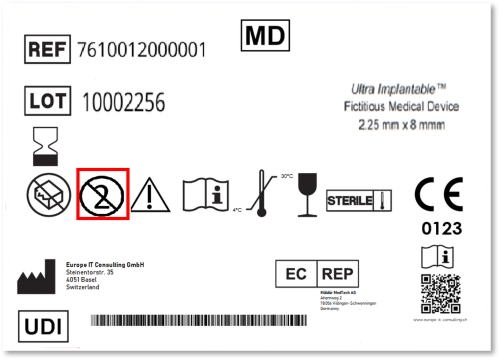
Since the EU Medical Device Regulation (MDR) came into force, the Unique Device Identification (UDI) system has become a cornerstone of regulatory compliance. For manufacturers of Single Use Devices (SUDs),…

The EU has designated several UDI issuing entities (Issuing Entities) to provide manufacturers with various options for generating and managing their Unique Device Identifiers (UDIs). The currently recognized entities are:…

What is a "New Device" according to MDR and IVDR? Introduction The Medical Device Regulation (MDR, EU 2017/745) and the In Vitro Diagnostic Regulation (IVDR, EU 2017/746) have significantly tightened…

What is an "Active Device" according to MDR and IVDR? Introduction The Medical Device Regulation (MDR, EU 2017/745) and In Vitro Diagnostic Regulation (IVDR, EU 2017/746) have tightened the requirements…

The live webinar has already ended, but you are very welcome to register to receive the recording and the presentation materials! The EUDAMED database plays a central role in MDR…