New EUDAMED FAQ

New EUDAMED FAQ: Comprehensive Answers to Your Questions About the EU Medical Device Database We are pleased to announce that we have expanded our website with a new, comprehensive FAQ…

New EUDAMED FAQ: Comprehensive Answers to Your Questions About the EU Medical Device Database We are pleased to announce that we have expanded our website with a new, comprehensive FAQ…

The importance of the EUDAMED packaging system for the regulation of medical devices in the EU The EUDAMED packaging system is an indispensable part of the broader EUDAMED system, which…

The EU Medical Device Regulation (MDR) introduced stricter rules and clearer responsibilities for all actors involved in the life cycle of medical devices. One area that raises particular questions is…
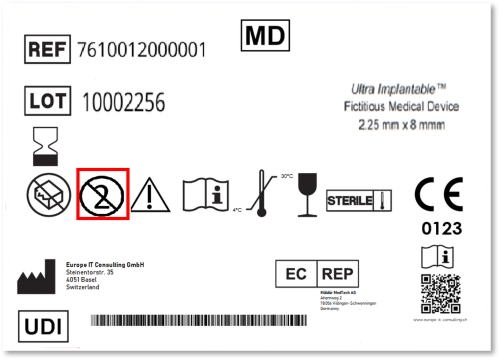
Since the EU Medical Device Regulation (MDR) came into force, the Unique Device Identification (UDI) system has become a cornerstone of regulatory compliance. For manufacturers of Single Use Devices (SUDs),…