
What Contact Lens Manufacturers Need to Know About the Master UDI-DI
At the end of August 2025, the Medical Device Coordination Group (MDCG) published its revised guidance MDCG 2024-14 Rev.1. This document addresses the practical implementation of the so-called Master UDI-DI specifically for contact lenses and provides manufacturers with clear direction on how to ensure regulatory compliance when applying this new identification concept. In addition to technical clarifications and refinements, the revision includes one major change: a new deadline for mandatory application—giving manufacturers more time to adapt their processes, IT systems, and labeling.
What Is the Master UDI-DI and Why Is It Important?
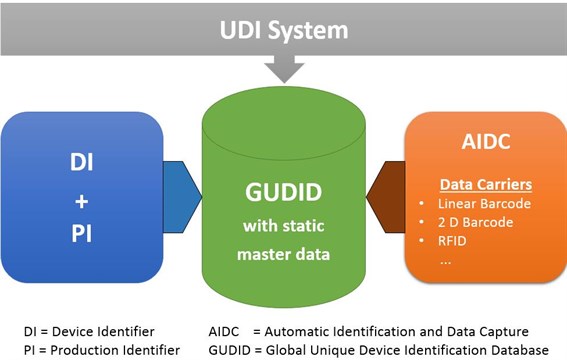
The Master UDI-DI is a newly introduced product identifier that allows highly similar variants of a medical device to be grouped under a shared identity—as long as they share certain clinically relevant design parameters. For contact lenses, which are typically marketed in hundreds of variants (e.g. different powers, base curves, or diameters), the Master UDI-DI significantly reduces the administrative and operational burden. Manufacturers no longer need to assign a unique UDI-DI to every individual power variant. Instead, they can group those variants under a single Master UDI-DI—provided they meet the grouping logic defined in the guidance.
At the same time, the Master UDI-DI becomes a central reference point for many regulatory processes. It will be printed on the product label and required in vigilance reports (e.g. for incidents or recalls) as well as during EUDAMED registration.
New Deadline: Mandatory as of 9 November 2026
The Delegated Regulation (EU) 2025/788 postponed the original deadline (May 2025) to 9 November 2026. This change gives the industry roughly 18 additional months to properly implement the Master UDI-DI framework.
However, the MDCG explicitly stresses that, even though the deadline was postponed, it is strategically advisable to start early. Especially in the context of vigilance and PMS processes, it is strongly recommended to begin assigning Master UDI-DIs now and to actively integrate them into systems such as ERP, PLM, or LIMS. This is the only way to ensure that affected products can be clearly identified and traced in the event of a serious incident.
Which Products Can Be Grouped Under a Master UDI-DI?
According to the new guidance, only contact lenses with identical clinical characteristics may be grouped under one Master UDI-DI. The MDCG defines the following mandatory parameters:
-
BC (Base Curve) – the base curvature of the lens
-
DIA (Diameter) – the overall diameter of the lens
Additional parameters may be required depending on clinical relevance. These may include:
-
Lens material (e.g. hydrogel vs. silicone hydrogel)
-
Lens type (spherical, toric, multifocal)
-
Filters or tinting
-
Front or back surface design
A manufacturer may, for example, group all spherical daily lenses with the same BC and DIA and identical material under a single Master UDI-DI—even if they differ in optical power (diopters). In contrast, toric lenses with the same BC and DIA but different materials must be assigned separate Master UDI-DIs if the material affects the clinical behavior of the product.
Impact on Product Labeling
A central element of the revised rule is that the Master UDI-DI will replace the UDI-DI on the product label for contact lenses. This means that individual power variants no longer require unique UDI-DIs on single-unit packaging (e.g. blister packs). Instead, the Master UDI-DI, combined with the UDI-PI (e.g. lot number, serial number, or manufacturing date), will be sufficient to ensure traceability of the specific item.
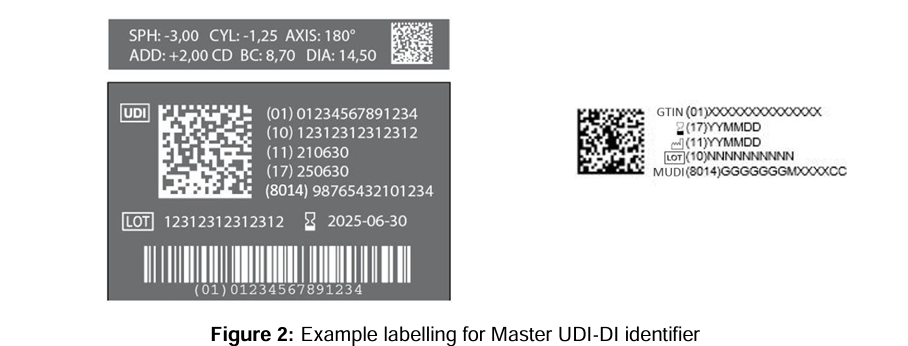
Quelle: MDCG 2024-14 Rev.1 – Guidance on the implementation of the Master UDI-DI solution for contact lenses
The labeling becomes more complex for multi-packs (e.g. 6-packs): if all included lenses have the same clinical parameters, one Master UDI-DI is sufficient. However, if BC or DIA vary across the pack, then separate packaging solutions or multiple Master UDI-DIs are required.
Relevance for Vigilance and EUDAMED
The MDCG clearly states that for vigilance reports, such as those related to side effects or product recalls, the full UDI (Master UDI-DI + UDI-PI) must be submitted. Identifying the device solely via the Basic UDI-DI or product name will no longer be accepted.
During the transition period—especially before the mandatory launch of the EUDAMED UDI/Device modules—an alternative traceability path using the EUDAMED Device ID + UDI-PI may still be used. Nevertheless, building a robust Master UDI-DI infrastructure is essential now to meet upcoming regulatory demands.
Conclusion: Now Is the Right Time to Act
Although the mandatory application of the Master UDI-DI for contact lenses has been postponed to 9 November 2026, the need for action is already here. The updated guidance provides clear criteria, practical examples, and a solid foundation for implementation—both technically and procedurally.
Manufacturers should use the extra time to:
-
structure their product data,
-
request Master UDI-DIs from an official issuing entity,
-
adapt their labeling and packaging processes,
-
and align their vigilance and EUDAMED procedures with the new requirements.
We have already published a separate news update regarding the revised timeline.
Taking early action not only ensures regulatory compliance—it also helps reduce internal workload related to data management and communication with authorities.
Source:







Related Posts