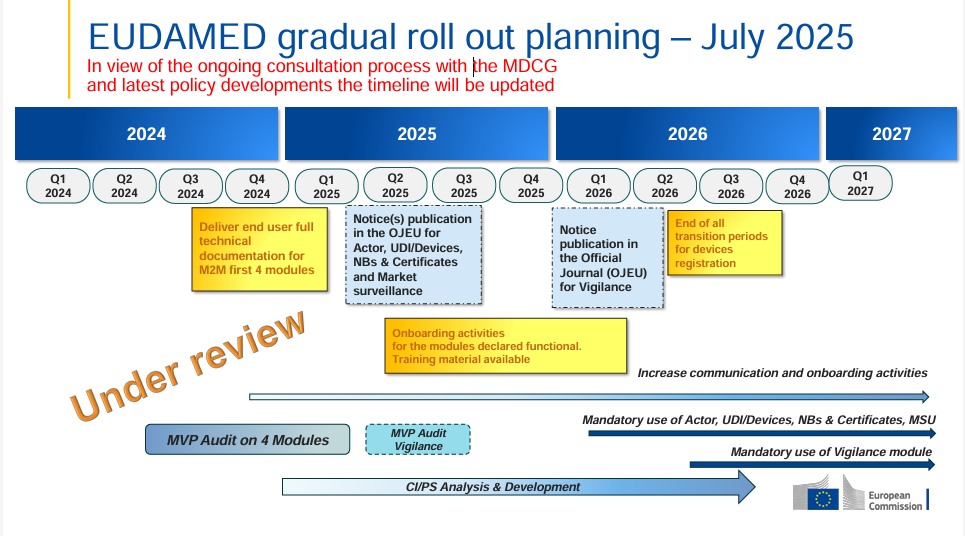
The European Commission has released an updated EUDAMED rollout plan (July 2025) – this time with a clear note: the timeline is now “under review”. This indicates that, as part of the ongoing MDCG consultation process, political and regulatory developments are currently being taken into account.
What’s new?
-
The “under review” label is now prominently displayed in the timeline – this was not the case in the January 2025 version.
-
The key milestones remain the same (OJEU notices for Actor, UDI/Devices, Notified Bodies & Certificates, Market Surveillance expected in Q2 2025; Vigilance module in Q4 2025; mandatory use from Q1 2026), but the review signals that changes are possible.
Why this matters
-
Manufacturers and stakeholders should closely monitor developments to be ready to adapt if deadlines shift.
-
The 2025 onboarding phase remains critical for testing, training, and technical preparations.
-
The possibility of deadline adjustments calls for flexible planning, but also for early readiness for mandatory use and training.









Related Posts