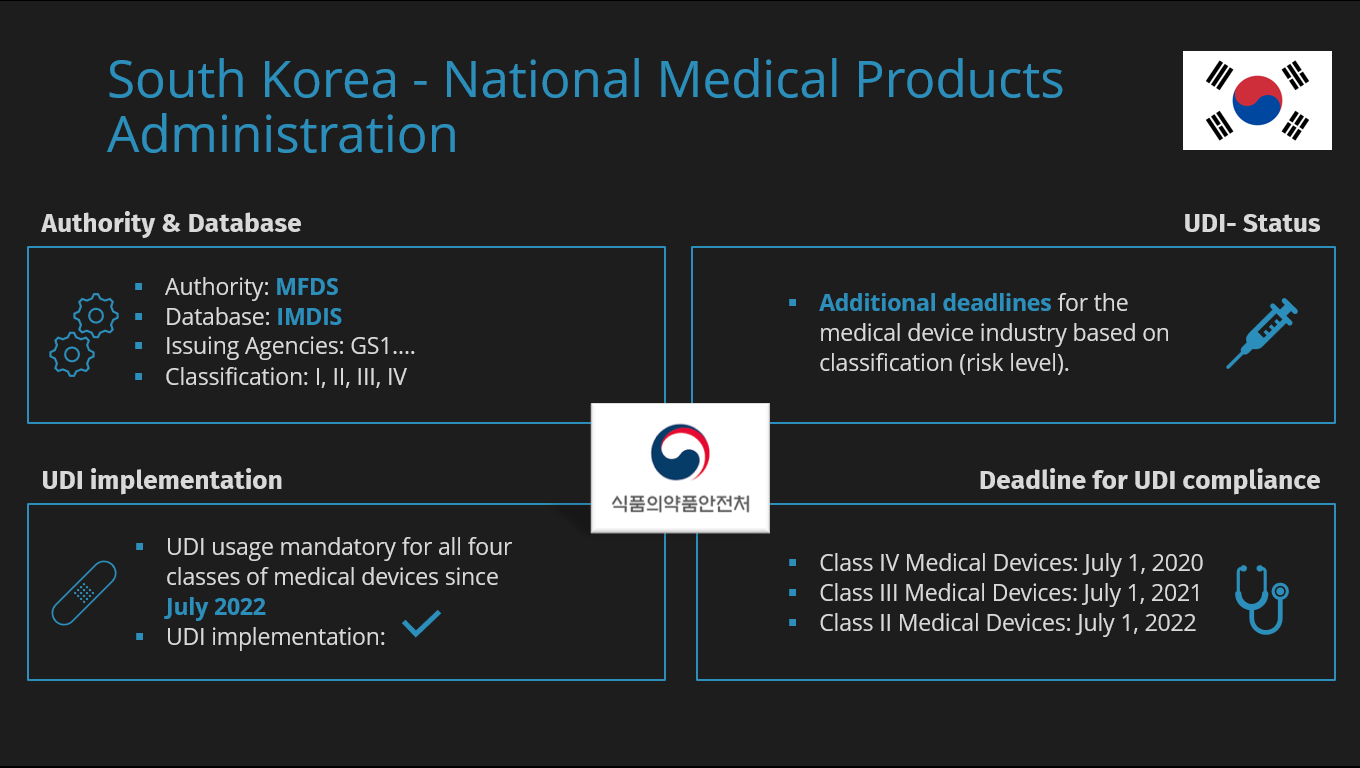
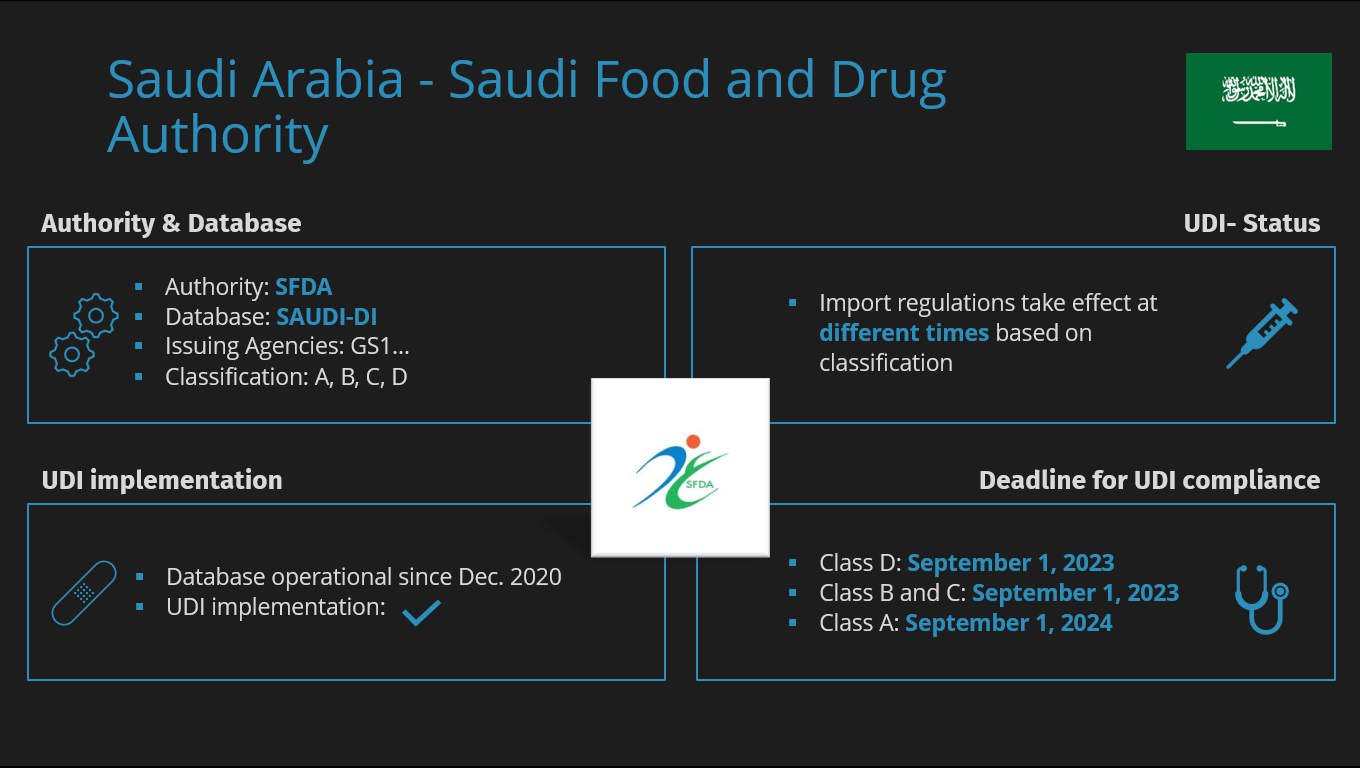
MDR causes shortages of medical devices

MDR causes shortages of medical devices The introduction of Unique Device Identification (UDI) means that manufacturers of medical devices are obligated to provide a comprehensive range of product information to…