Global Submission Portal:
The New Era of Regulatory Compliance

A unified platform for global FDA and EUDAMED submissions is now available
Europe IT Consulting is pleased to announce the launch of the Global Submission Portal – a groundbreaking solution that simplifies and accelerates regulatory compliance processes for medical device manufacturers worldwide.
The Challenge
Medical device manufacturers face the complex task of registering and reporting their products to different authorities worldwide. Each authority has its own requirements, formats and processes. This leads to:
- Zeitaufwändigen manuellen Prozessen
- Time-consuming manual processes
- High risk of errors during data entry
- Lack of transparency regarding submission status
- Fragmented workflows across multiple systems
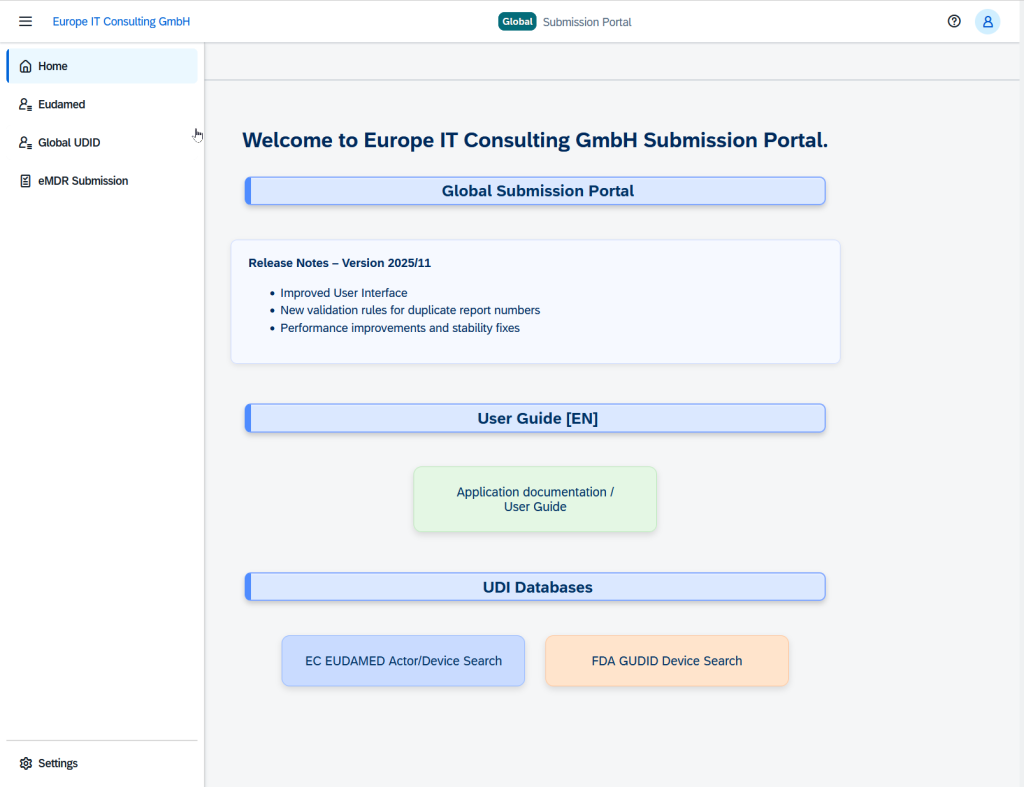
The Solution: Global Submission Portal
Our Global Submission Portal integrates multiple submission workflows into a single, user-friendly platform. Manufacturers and regulatory teams can now transmit compliance-relevant data sets directly to authorities worldwide – efficiently, securely and transparently.
Core Functionality
The portal automates the entire submission process: from Excel-based data collection and automatic validation through to direct transmission to the authorities – all in one seamless workflow.
Supported Modules
The portal currently supports four critical compliance areas:
- 🇺🇸 FDA eMDR
Electronic Medical Device Reporting for fast FDA reporting - 🇺🇸 FDA GUDID
Global UDI Database for US market registration - 🇪🇺 EUDAMED UDI
EU MDR-compliant UDI submissions - 🇪🇺 EUDAMED Vigilance
EU Manufacturer Incident Reports
Key Features in detail
Excel-based Upload
Simply upload your prepared Excel files. The system automatically recognizes the data structure and prepares the submission. No complex forms, no repeated data entry.
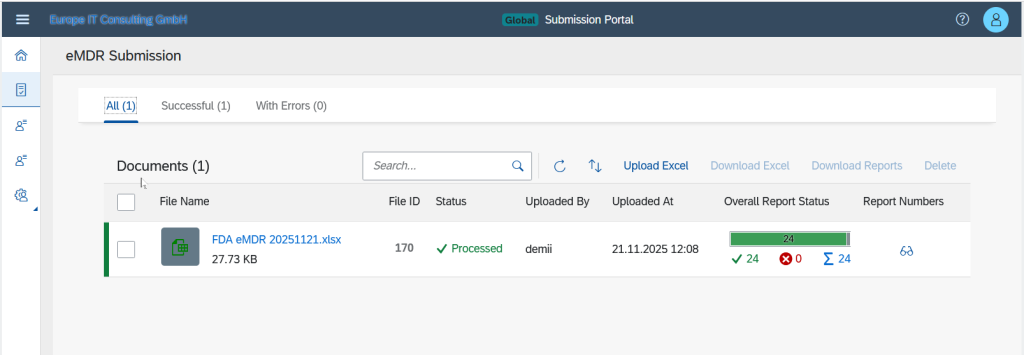
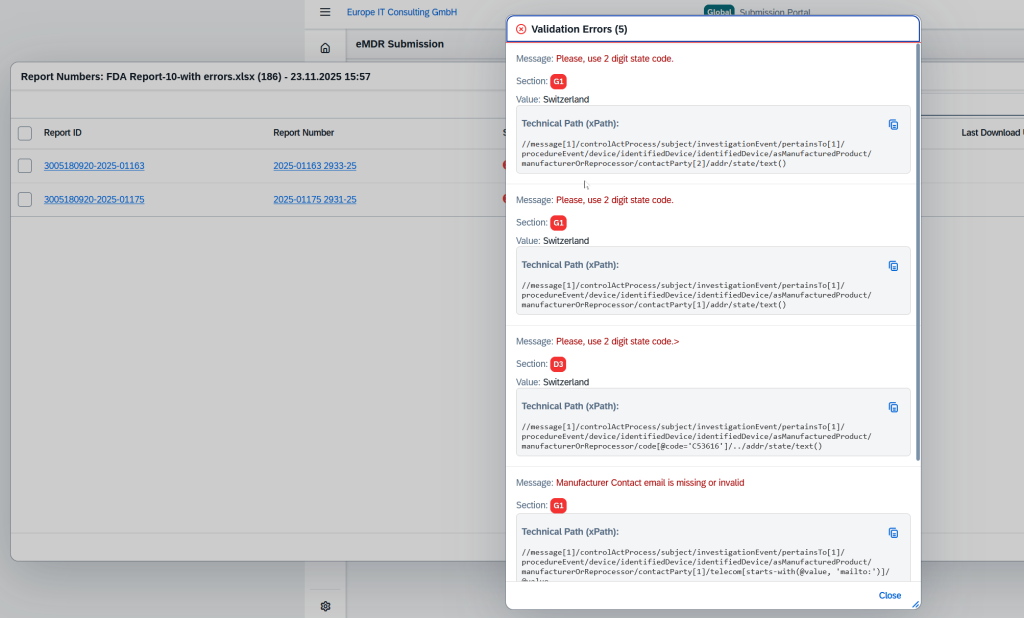
Validation Errors
For failed submissions, detailed validation errors are displayed, including the section, the exact XPath location and the specific authority message.
Real-time Status Tracking
Each submission passes through transparent status stages:
- Uploaded – File uploaded, waiting for processing
- Processing – Validation and transmission to the authority in progress
- Success – Successfully accepted by the authority
- Failed – Validation or submission failed
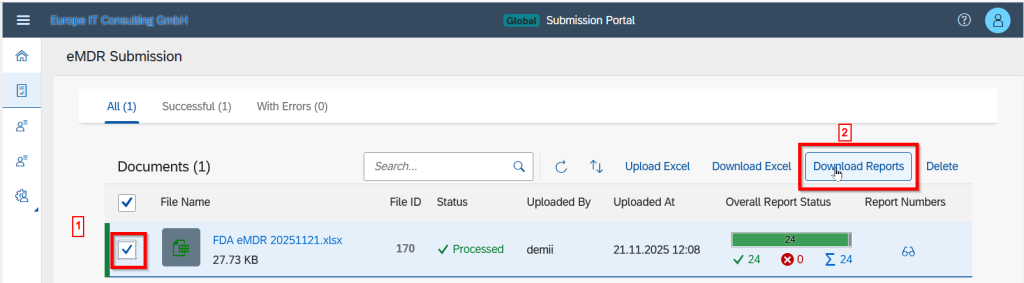
Comprehensive Download Options
After processing, all result files are available for download – either as a complete ZIP package or as individual reports. Authority responses (e.g. FDA ACK2) are displayed directly in the portal and are included in the ZIP archive.
Smart Notifications
Configure email notifications to be informed as soon as your submissions have been processed. Stay up to date without having to constantly check the portal.
Important Note on Data Availability
All files remain accessible for 6 months before they are automatically deleted. Make sure to download all required documents before this period expires.
Security and Compliance
The Global Submission Portal adheres to the highest security standards:
- Encrypted data transmission (TLS/SSL)
- Strict data isolation per customer
- Access controls and user rights management
- Audit trail for all activities
- Regular security audits
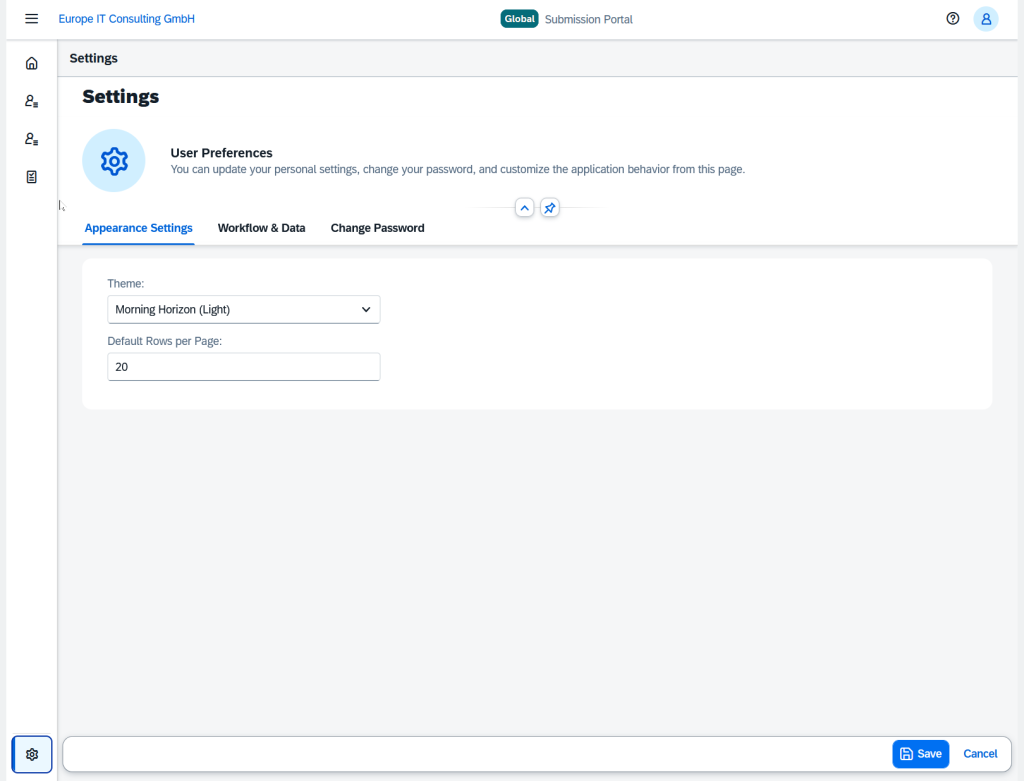
Customizable Settings
Via the settings page, users can configure the portal according to their needs:
- Look & feel and theme customization
- Workflow preferences
- Notification settings
- Password management with “forgot password” function
Continuous Enhancement
The Global Submission Portal is continuously updated to support new regulatory requirements. Release notes and new features are communicated directly within the platform.
Planned enhancements include additional international authorities and further compliance modules to optimally support our customers worldwide.
Global Submission Portal
With the Global Submission Portal, Europe IT Consulting provides a modern, efficient solution to the challenges of global regulatory compliance. The combination of user-friendliness, automation and security makes the portal the ideal choice for medical device manufacturers who want to optimize their compliance processes.
Experience for yourself how the Global Submission Portal can revolutionize your regulatory workflows!