MDCG Publishes Timelines for the Introduction of the Master UDI-DI for Eyewear and Contact Lenses
The Medical Device Coordination Group (MDCG) has released its MDCG 2025-7 position paper, setting out the official timelines for the implementation of the Master UDI-DI for highly individualised medical devices, including:
-
Contact lenses
-
Spectacle frames
-
Spectacle lenses
-
Ready-to-wear reading spectacles
Background
The Master UDI-DI is a new identification concept for highly individualised products. It allows similar devices with identical design characteristics to be grouped under a single identifier in EUDAMED.
The aim is to reduce the number of UDI records and simplify management for manufacturers, distributors, and authorities.
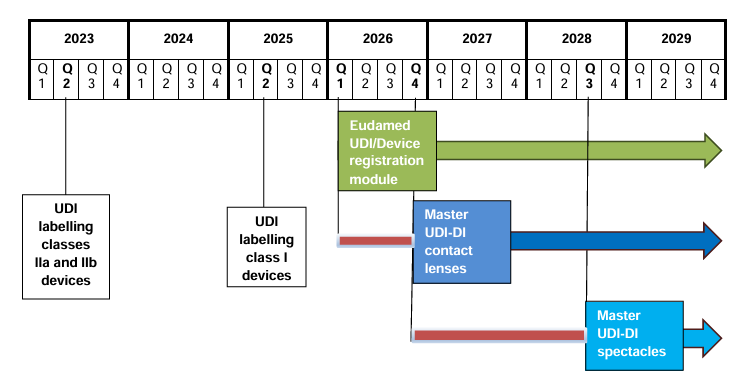
Key Deadlines at a Glance
| Product Category | UDI Labelling Requirement | Mandatory Use of EUDAMED UDI/Device Registration Module | Mandatory Master UDI-DI | Transition Phase |
|---|---|---|---|---|
| Contact lenses | 26 May 2023 (Class IIa/IIb) | Q1 2026 | 9 November 2026 | Q1 2026 – Q4 2026 |
| Spectacle frames, spectacle lenses, ready-to-wear reading spectacles | 26 May 2025 (Class I) | Q1 2026 | September 2028 | Q1 2026 – Q3 2028 |
Voluntary Implementation Encouraged
Manufacturers are allowed – and encouraged – to assign Master UDI-DIs before the mandatory deadlines. Early adoption offers benefits such as:
-
Gaining operational experience with the new identifier
-
Facilitating vigilance reporting and post-market surveillance
-
Ensuring internal processes are fully aligned before deadlines
Industry Impact
The introduction of the Master UDI-DI marks a significant step toward harmonisation and reduced administrative burden in UDI management. However, manufacturers should start preparing now to ensure technical readiness and regulatory compliance.
Further resources:








Related Posts