
We are thrilled by the enormous interest in our free expert webinar on the EUDAMED database! With over 700 registrations, we clearly felt how relevant and important this topic is for the medical device industry.
📈 This shows us:
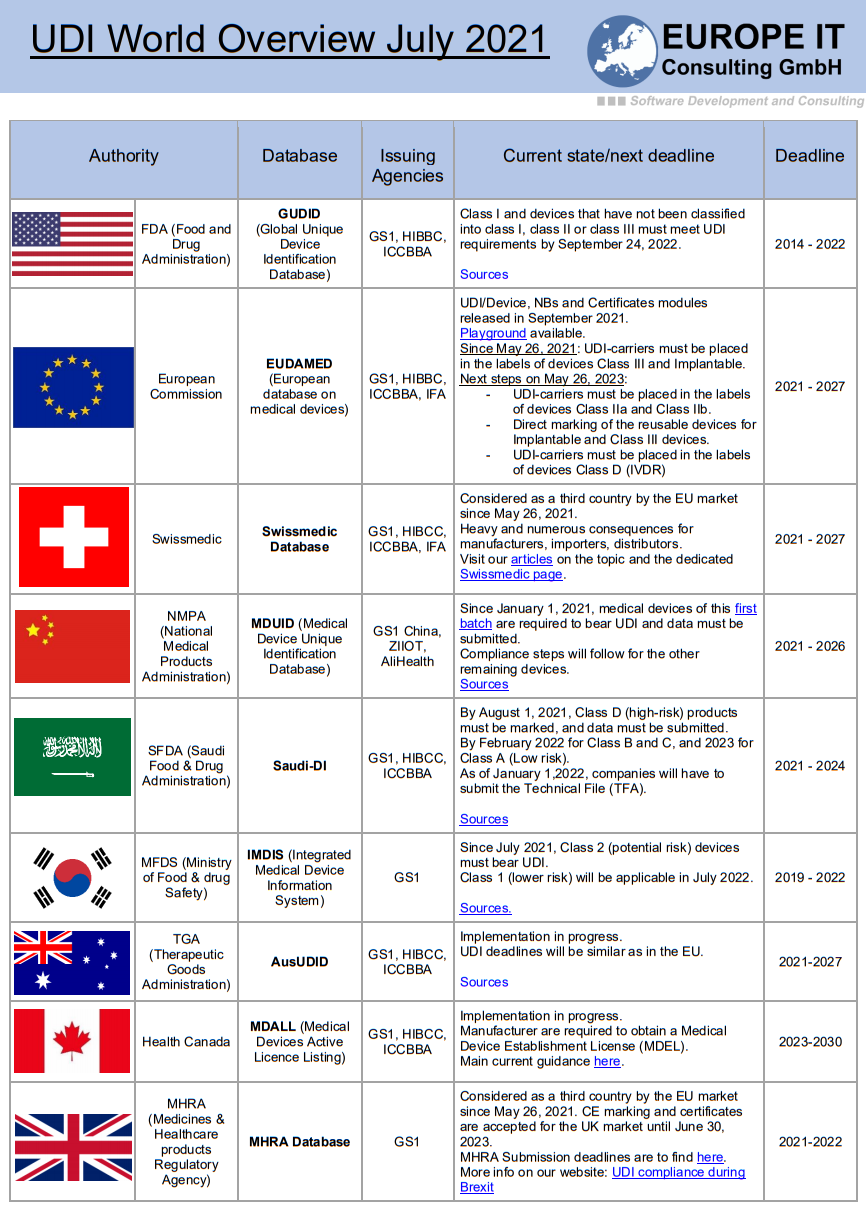
EUDAMED compliance concerns many manufacturers, importers, and distributors of medical devices – and the need for information is huge!
🎬 Did you miss the live webinar? No problem!
Due to the fantastic response, we are happy to continue providing the complete webinar recording as well as all accompanying materials.
Register now and get immediate access to:
✅ The complete webinar recording (approx. 60 minutes)
✅ Presentation slides in PDF
✅ Whitepaper
What to expect in the recording:
🔸 Status & Timeline – Current EUDAMED modules and critical deadlines
🔸 EMDN Codes & SRN – Clear explanation of key concepts
🔸 Practical Platform Guide – Step-by-step through the EUDAMED website
🔸 Data Submission – Efficient and secure upload strategies
🔸 Expert Answers – Frequently asked questions from the live session
💡 Your advantage: Review the content at your own pace and use the materials as a reference guide for your EUDAMED compliance strategy.
Register for free now and get immediate access! 👆








Related Posts