The full UDI lifecycle – everything from a single source
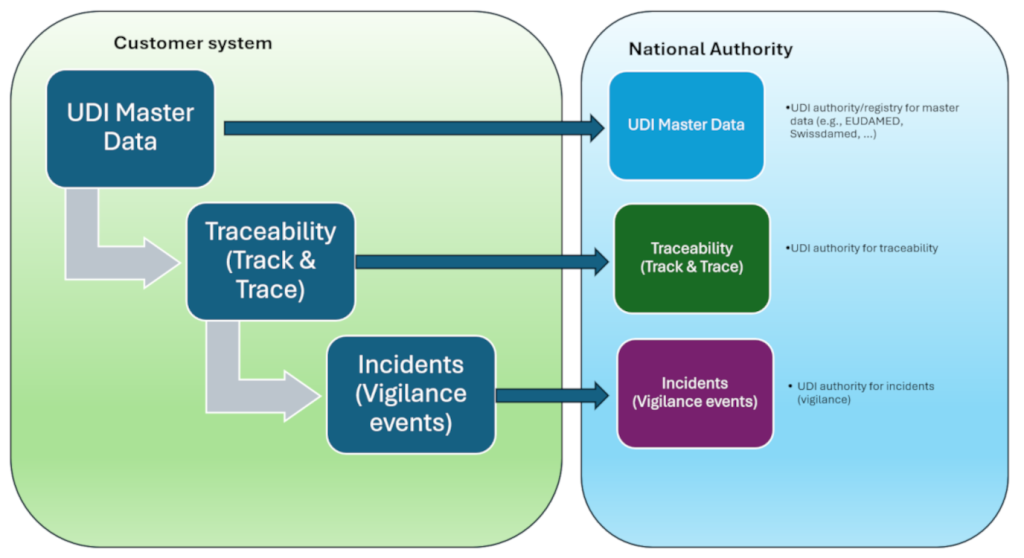
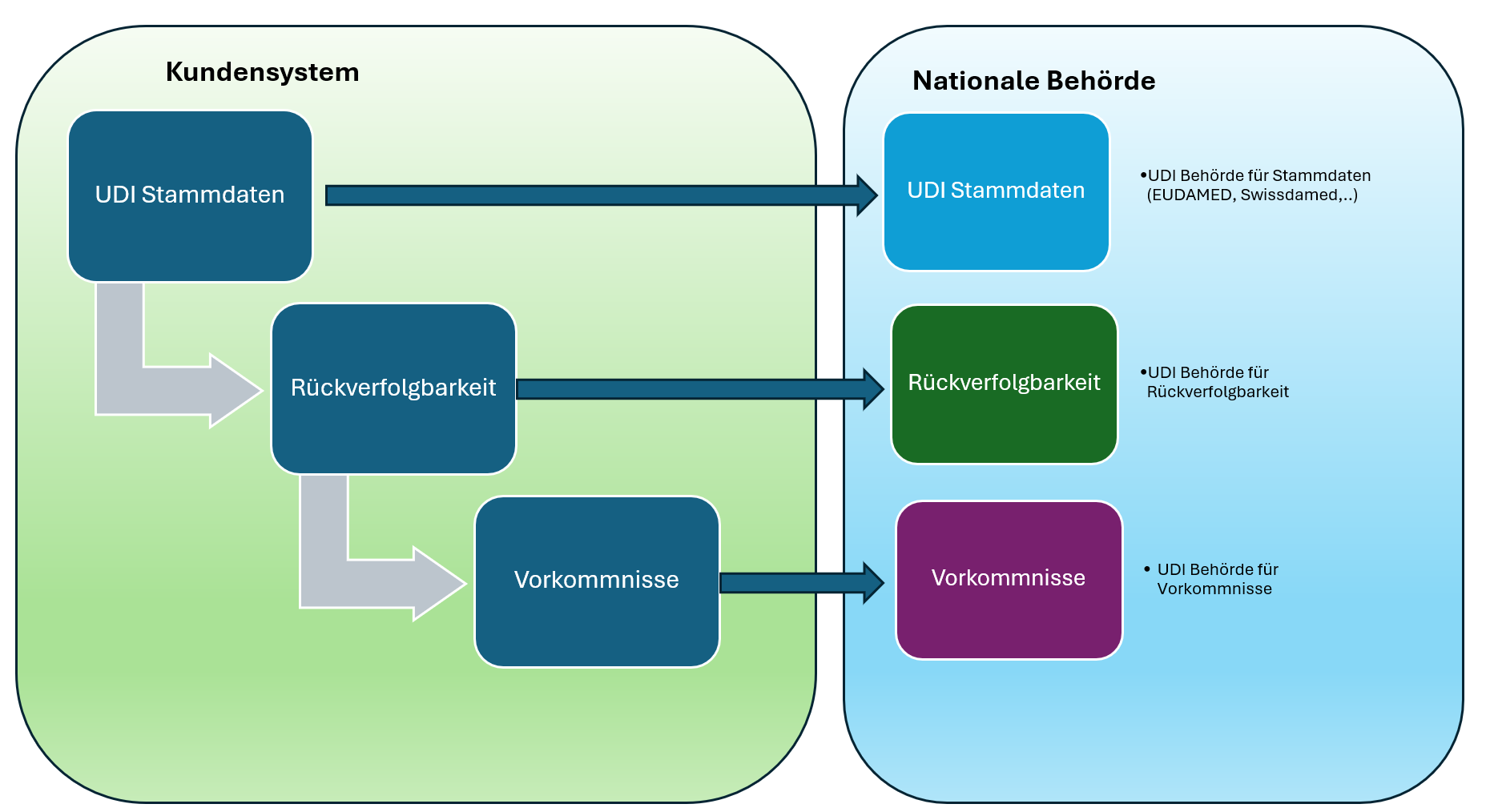
The path to full UDI compliance can be understood as a data cycle:
UDI master data → Traceability → Vigilance
Each building block matters, but only their interaction creates tangible value for manufacturers, authorities, and patients.
It starts with UDI master data registration with the competent authorities (GUDID, EUDAMED, swissdamed, etc.).
This includes the UDI-DI (e.g., GTIN under GS1), the Basic UDI-DI (EU), and the UDI-PI (e.g., lot/serial, manufacturing/expiry date). These data are validated, versioned, and submitted to the relevant registries/authorities—forming the basis for transparency, market access, and consistent labeling/packaging hierarchies.
This is followed by product traceability. Only when master data and GTIN are seamlessly linked can products be uniquely identified across the entire lifecycle—from goods receipt through to clinical use. This bridge ensures that regulatory requirements are not only fulfilled on paper but also work reliably in day-to-day operations.
If an incident occurs—such as an adverse event or a recall—the loop closes. Through systems like eMDR or ICSR reports, the data are transmitted securely and in compliance to the authorities. This is where the true value of the upstream processes becomes clear: only well-maintained master data and clear traceability enable rapid and error-free reporting.
We cover the entire UDI lifecycle and have translated it into proven, practical solutions.
For the initial master-data registration, customers maintain their UDI master data via our Global UDI Add-on centrally or using the UDI Excel Template, validate them with rule-based checks, and submit them to UDI authorities worldwide.
For traceability, we provide integration components that transmit manufacturing information as well as serial/lot movements and sales to customers directly from the ERP to government track-and-trace systems—for example, Türkiye’s ÜTS.
Incidents can be submitted in bundled form to the responsible national authorities using our Excel template or directly from the SAP Fiori application.
We are happy to assess your status quo and outline suitable options. Get in touch—our advice is pragmatic and non-binding.







Related Posts