Maximum number of reuses of device

Information on the maximum number of reuses of devices and their implications on UDI for MDR 2017/745 compliance was recently provided by the MDCG in the Guidance on BASIC UDI-DI…

Information on the maximum number of reuses of devices and their implications on UDI for MDR 2017/745 compliance was recently provided by the MDCG in the Guidance on BASIC UDI-DI…

The IVDR provisions must be applied before May 26, 2022 (during the transition period). As obscurity still remains, the Medical Device Coordination Group (MDCG) recently published indications on how to deal…
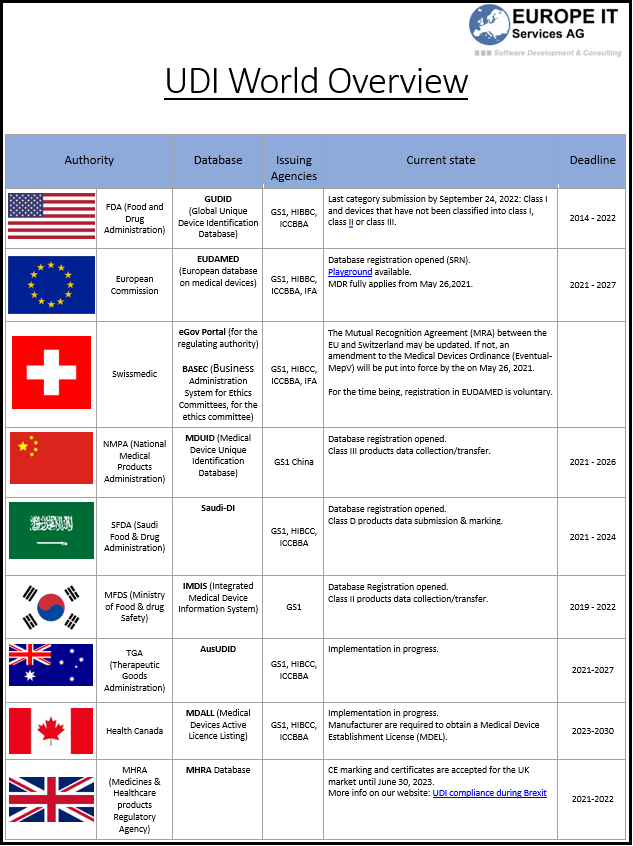
The UDI (Unique Device Identification) field is still evolving and is more or less advanced depending on the country. If you are in any way related to the medical devices…

Before jumping headfirst into the complexity of the Medical Device Regulation, you must be 100% sure that it is worth it. This assessment can be complex if you sell health-related…
The variety of medical devices is also expressed in their different forms. These are an important factor influencing the legal status and the way in which the medical device is…

The UK's exit from the European Union has a great impact on many companies and the medical device business. The following article summarizes the upcoming changes and explains what to…

The European Medical Devices Database (EUDAMED) is not expected to be operational and fully functional with all its 6 modules before May 2022. Nevertheless, the effective date of the Medical…

The process of UDI implementation according to EUDAMED and/or FDA requirements is time-consuming and costly. However, there are significant benefits that go far beyond mere compliance. As a manufacturer and/or…
The format of your UDI codes, Basic UDI-DI and UDI-DI, depends on the selected Issuing Agency. For the two most commonly used Issuing Agencies, GS1 and HIBC, we have summarized…

On the 8th February 2021 the European Commission published a Guidance on the Management of the Legacy Devices. It explains in detail how Legacy Devices will be identified and the…